Korean J Gastroenterol.
2014 Jun;63(6):341-347. 10.4166/kjg.2014.63.6.341.
Difficult Establishment of a Chronic Nonsteroidal Anti-inflammatory Drugs Induced Gastric Inflammation Rat Model due to Gastric Adaptation and Small Bowel Damage
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. nayoungkim49@empas.com
- 2Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Incheon Sarang Hospital, Incheon, Korea.
- 4Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2234034
- DOI: http://doi.org/10.4166/kjg.2014.63.6.341
Abstract
- BACKGROUND/AIMS
The prevalence of peptic ulcer disease has not decreased mainly due to an increase in the use of NSAIDs. This study was conducted in order to determine whether a chronic NSAID-induced gastric inflammation model could be established by repeated administration of NSAID.
METHODS
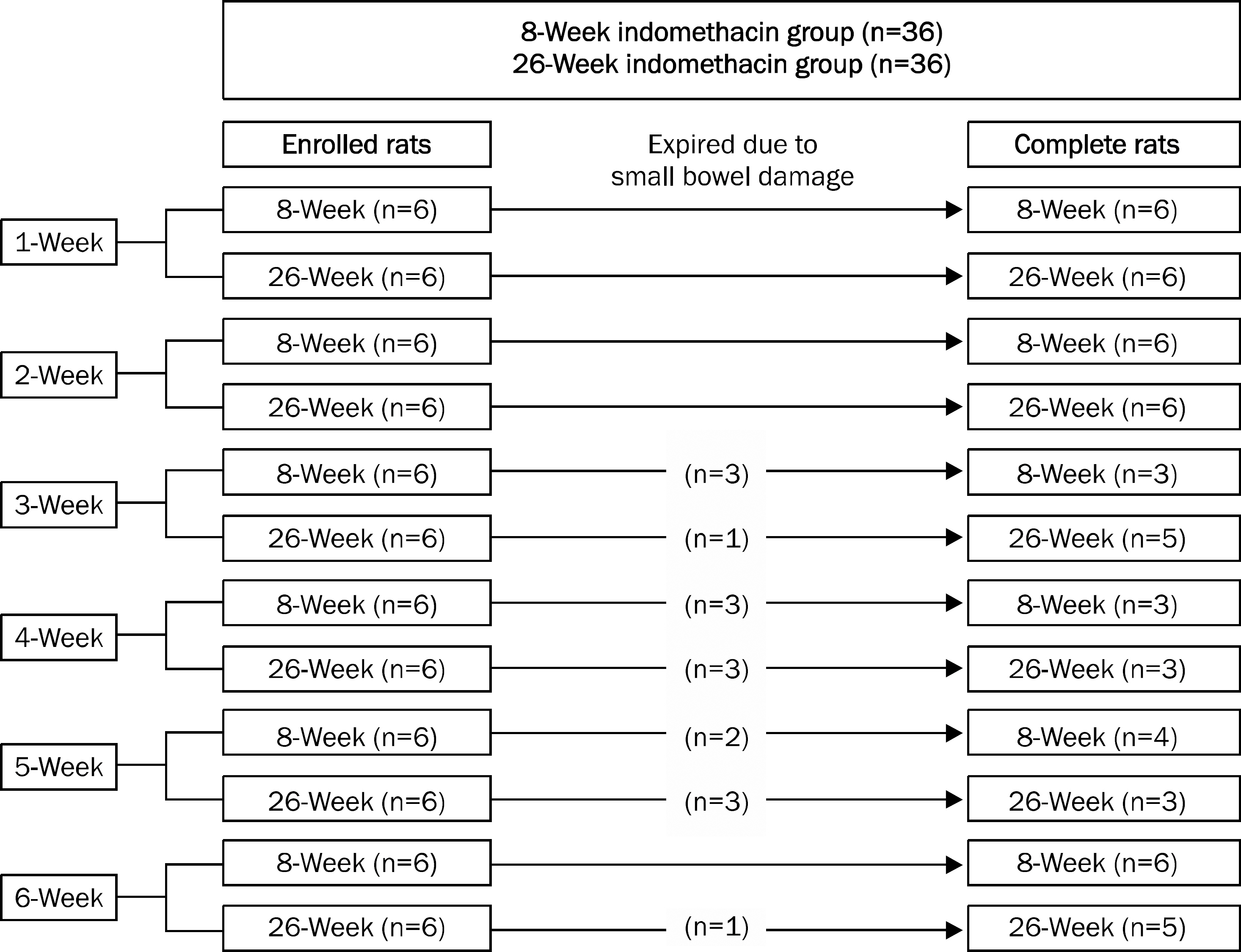
Indomethacin (10 mg/kg) was administered once per week for six weeks in 8- and 26-week rats and animals were sacrificed every week after administration. Gross ulcer index, histologic damage index, myeloperoxidase (MPO) activity, and mucus (glucosamine) levels were measured. Small bowel damage was also evaluated.
RESULTS
Gross gastric damage index showed a peak level at three weeks and then decreased slowly in the 26-week indomethacin group. Gastric mucosal glucosamine level increased in both the 8-week (p=0.038) and 26-week groups (p=0.007). In addition, gastric mucosal MPO level decreased in the 8-week group (p=0.018) but did not show a decrease in the 26-week group. Small bowel damage began to occur at three weeks during the schedule and eight of 36 rats (22.2%) died due to perforation or peritonitis of the small bowel in the 8- and 26-week indomethacin groups, respectively.
CONCLUSIONS
Due to gastric adaptation and small bowel damage, repeated administration of NSAID to experimental animals may not be an adequate method for establishment of the chronic gastric inflammation model.
MeSH Terms
-
Animals
Anti-Inflammatory Agents, Non-Steroidal/*toxicity
Disease Models, Animal
Gastric Mucosa/*drug effects/enzymology/pathology
Glucosamine/metabolism
Indomethacin/*toxicity
Intestine, Small/*drug effects/pathology
Male
Peroxidase/metabolism
Rats
Rats, Sprague-Dawley
Time Factors
Anti-Inflammatory Agents, Non-Steroidal
Glucosamine
Indomethacin
Peroxidase
Figure
Cited by 1 articles
-
Nonsteroidal Anti-inflammatory Drug and Aspirin-induced Peptic Ulcer Disease
Young Kwang Shim, Nayoung Kim
Korean J Gastroenterol. 2016;67(6):300-312. doi: 10.4166/kjg.2016.67.6.300.
Reference
-
References
1. Wong GL, Wong VW, Chan Y, et al. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009; 137:525–531.2. Gisbert JP, Calvet X. Review article: Helicobacter pylori-negative duodenal ulcer disease. Aliment Pharmacol Ther. 2009; 30:791–815.3. Sung JJ, Chan FK, Chen M, et al. Asia-Pacific Working Group. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011; 60:1170–1177.
Article4. Yang YX, Lewis JD, Epstein S, Metz DC. Longterm proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296:2947–2953.
Article5. Okabe S, Pfeiffer CJ. Chronicity of acetic acid ulcer in the rat stomach. Am J Dig Dis. 1972; 17:619–629.
Article6. Kang JM, Kim N, Kim B, et al. Enhancement of gastric ulcer healing and angiogenesis by cochinchina Momordica seed extract in rats. J Korean Med Sci. 2010; 25:875–881.
Article7. Evangelista S. Role of sensory neurons in restitution and healing of gastric ulcers. Curr Pharm Des. 2006; 12:2977–2984.
Article8. Fiorucci S, Distrutti E, de Lima OM, et al. Relative contribution of acetylated cyclooxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. FASEB J. 2003; 17:1171–1173.
Article9. Polat B, Suleyman H, Alp HH. Adaptation of rat gastric tissue against indomethacin toxicity. Chem Biol Interact. 2010; 186:82–89.
Article10. Nam SY, Kim N, Lee CS, et al. Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005; 50:2110–2120.
Article11. Lacy ER, Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982; 83:619–625.
Article12. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982; 78:206–209.
Article13. Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990; 186:729–742.14. Nishida K, Ohta Y, Ishiguro I. Teprenone, an anti-ulcer agent, increases gastric mucosal mucus level via nitric oxide in rats. Jpn J Pharmacol. 1998; 78:519–522.15. Alderman BM, Cook GA, Familari M, Yeomans ND, Giraud AS. Resistance to apoptosis is a mechanism of adaptation of rat stomach to aspirin. Am J Physiol Gastrointest Liver Physiol. 2000; 278:G839–G846.
Article16. Konturek SJ, Brzozowski T, Stachura J, Dembinski A, Majka J. Role of gastric blood flow, neutrophil infiltration, and mucosal cell proliferation in gastric adaptation to aspirin in the rat. Gut. 1994; 35:1189–1196.
Article17. Konturek PC, Brzozowski T, Pierzchalski P, et al. Activation of genes for spasmolytic peptide, transforming growth factor alpha and for cyclooxygenase (COX)-1 and COX-2 during gastric adaptation to aspirin damage in rats. Aliment Pharmacol Ther. 1998; 12:767–777.
Article18. Kuroda M, Yoshida N, Ichikawa H, et al. Lansoprazole, a proton pump inhibitor, reduces the severity of indomethacin-induced rat enteritis. Int J Mol Med. 2006; 17:89–93.
Article19. Saud B, Nandi J, Ong G, Finocchiaro S, Levine RA. Inhibition of TNF-alpha improves indomethacin-induced enteropathy in rats by modulating iNOS expression. Dig Dis Sci. 2005; 50:1677–1683.20. Stadnyk AW, Dollard C, Issekutz TB, Issekutz AC. Neutrophil migration into indomethacin induced rat small intestinal injury is CD11a/CD18 and CD11b/CD18 co-dependent. Gut. 2002; 50:629–635.
Article21. Ogihara Y, Okabe S. Mechanism by which indomethacin delays gastric ulcer healing in the rat: inhibited contraction of the ulcer base. Jpn J Pharmacol. 1993; 61:123–131.
Article22. Penney AG, Malcontenti-Wilson C, O'Brien PE, Andrews FJ. NSAID-induced delay in gastric ulcer healing is not associated with decreased epithelial cell proliferation in rats. Dig Dis Sci. 1995; 40:2684–2693.
Article23. Wang JY, Yamasaki S, Takeuchi K, Okabe S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology. 1989; 96:393–402.
Article24. Onodera S, Tanaka M, Aoyama M, et al. Antiulcer effect of lafuti-dine on indomethacin-induced gastric antral ulcers in refed rats. Jpn J Pharmacol. 1999; 80:229–235.
Article25. Satoh H, Inada I, Hirata T, Maki Y. Indomethacin produces gastric antral ulcers in the refed rat. Gastroenterology. 1981; 81:719–725.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Neutrophil on Nonsteroidal Anti-Inflammatory Drugs Induced Acute Gastric Mucosal Injury
- Helicobacter pylori-associated Chronic Atrophic Gastritis and Progression of Gastric Carcinogenesis
- The Effects of Helicobacter pylori Infection on Nonsteroidal Anti-inflammatory Drugs-induced Gastroduodenopathy
- Nonsteroidal Anti-inflammatory Drugs
- Gastroprotective Effect of Cochinchina momordica Seed Extract in Nonsteroidal Anti-Inflammatory Drug-Induced Acute Gastric Damage in a Rat Model