J Korean Ophthalmol Soc.
2009 Oct;50(10):1563-1568. 10.3341/jkos.2009.50.10.1563.
Effect of High Glucose on the Oxidative Stress in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu College of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2212776
- DOI: http://doi.org/10.3341/jkos.2009.50.10.1563
Abstract
- PURPOSE
To investigate the effect of high glucose (HG) on the oxidative stress in cultured human trabecular meshwork cells (HTMC).
METHODS
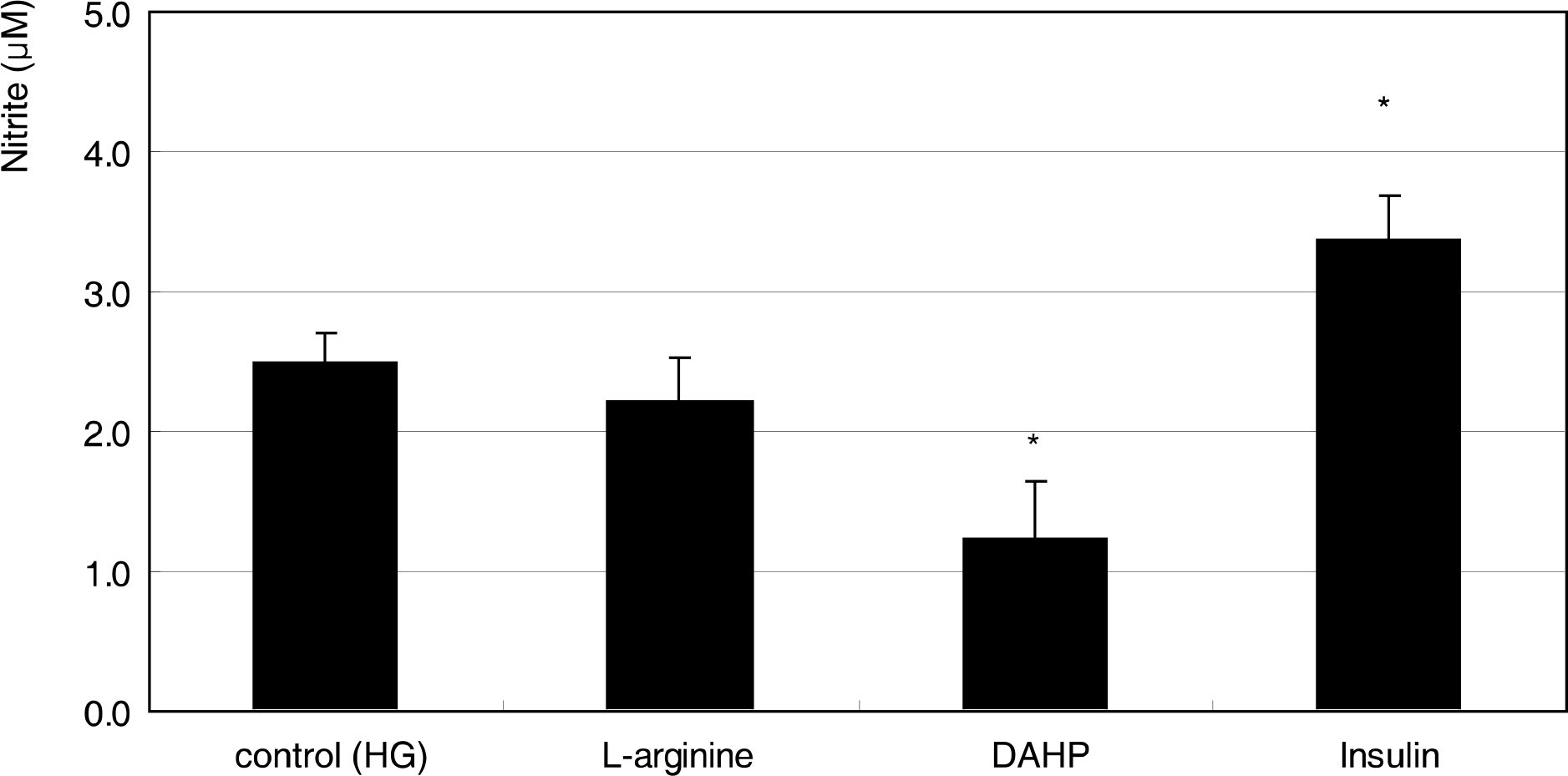
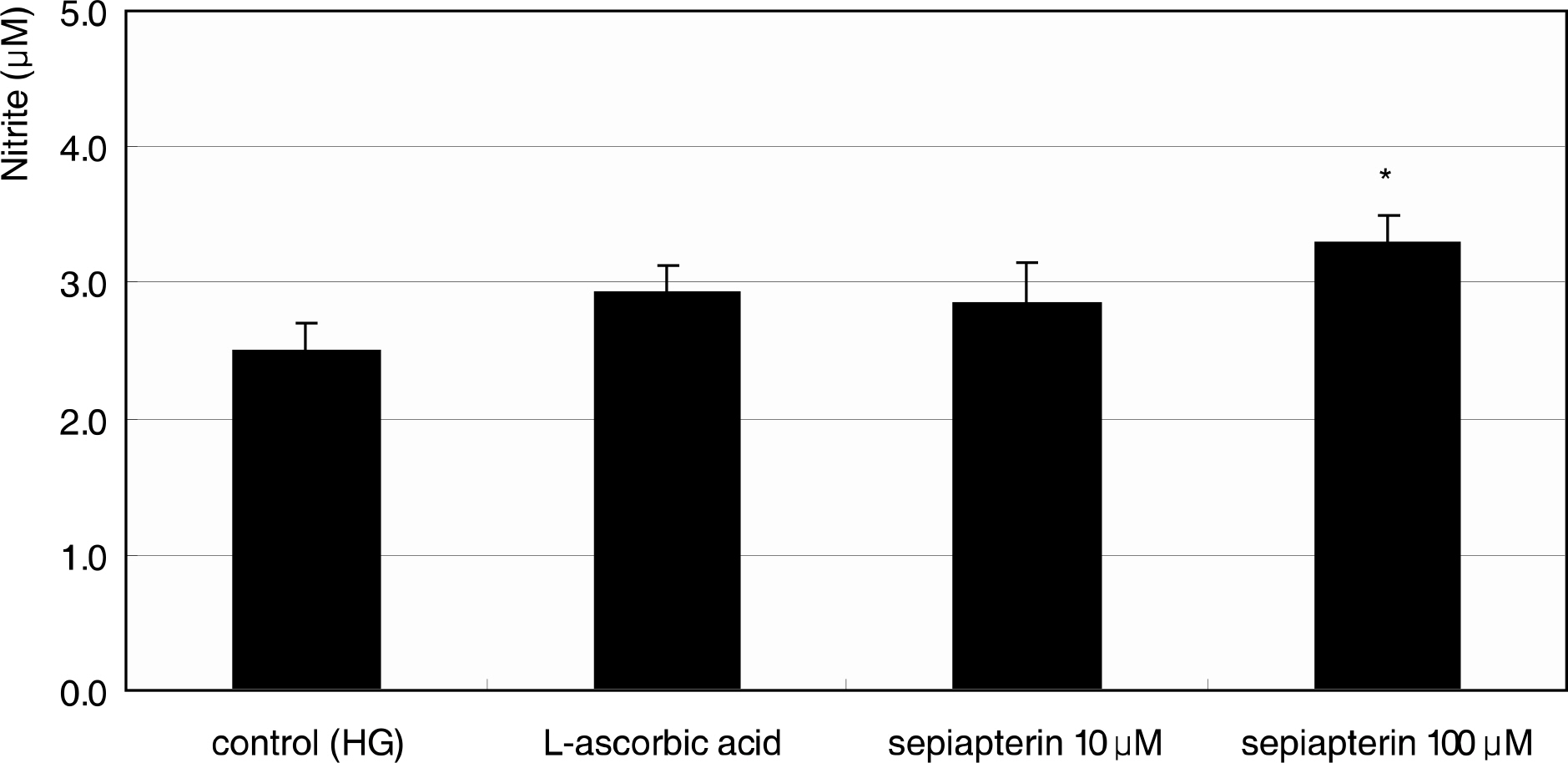
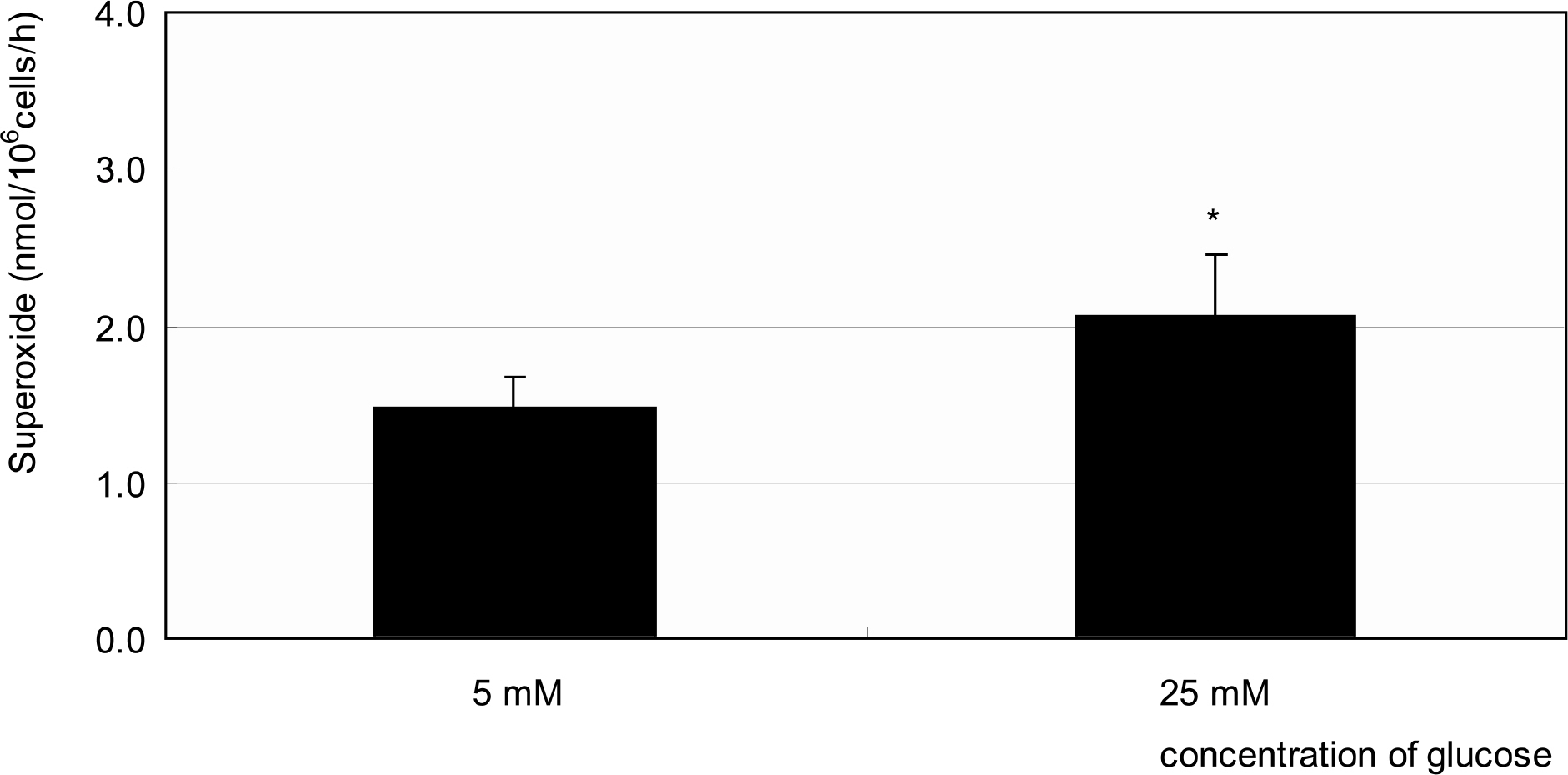
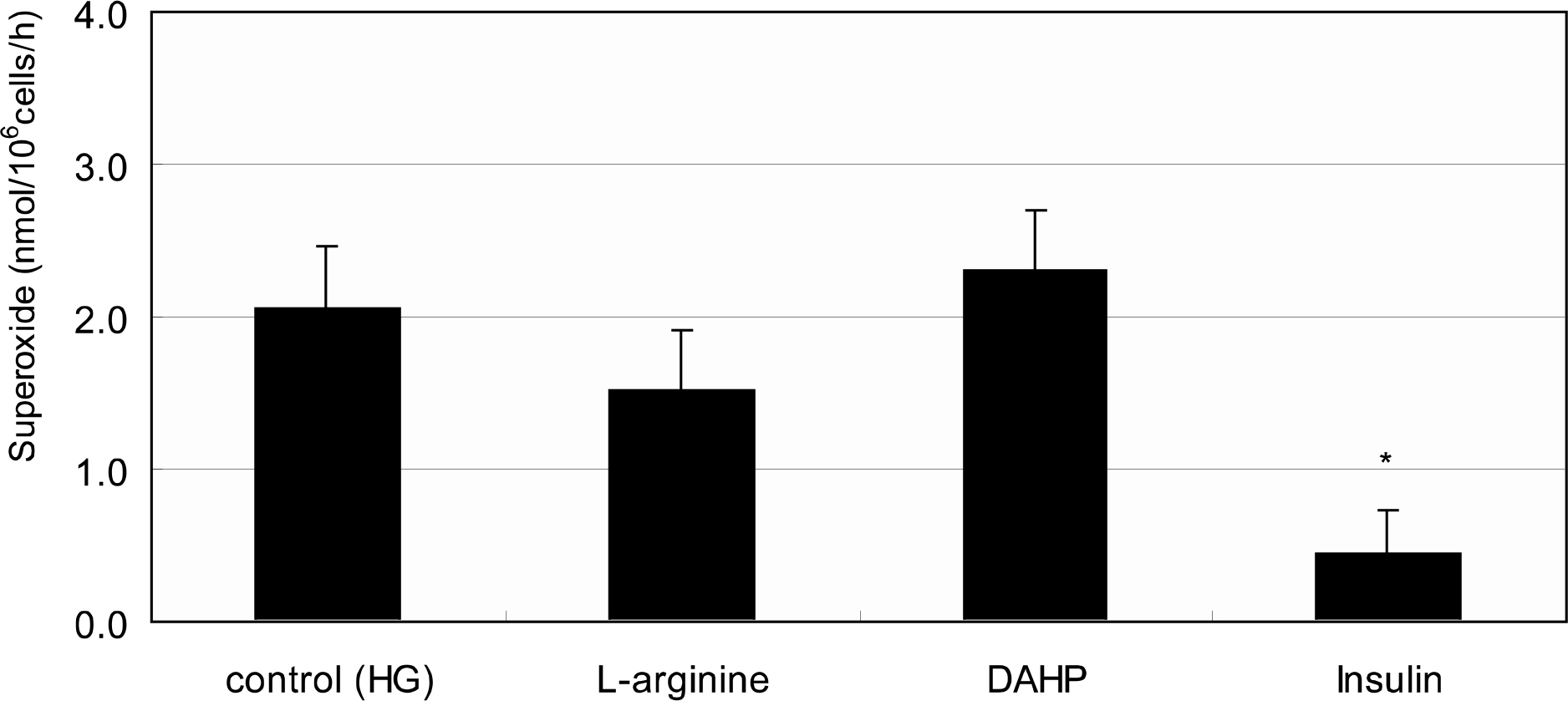
Primarily cultured HTMC were exposed to low glucose (5 mM) and HG (25 mM) for 7 days. Additionally, 1 mM L-arginine, 5 mM DAHP, 10 microgram/ml insulin, 100 micrometer L-ascorbic acid, 10, and 100 micrometer sepiapterin were co-exposed. The cellular survival and nitric oxide (NO) production were assessed by MTT assay and Griess assay, respectively. Superoxide production was measured by modified cytochrome c assay.
RESULTS
HG did not affect the survival of cultured HTMC significantly. HG decreased NO production. Co-exposed DAHP decreased but DAHP and insulin increased NO production. In addition, HG increased superoxide production, which was decreased by insulin, L-ascorbic acid, and sepiapterin.
CONCLUSIONS
HG decreased NO production accompanied with increased superoxide production in HTMC. Thus HG induces oxidative stress in HTMC and may cause cellular dysfunction and damage of the trabecular meshwork.
MeSH Terms
Figure
Reference
-
References
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.
Article2. Rohen JW, LÜtjen-drecoll E, FlÜgel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma. Exp Eye Res. 1993; 56:683–92.3. Alvarado JA, Alvarado RG, Yeh RF, et al. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells. Br J Ophthalmol. 2005; 89:1500–5.
Article4. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.5. Wana RF, Podos SM. Effect of the topical application of nitro-glycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.6. Nathanson JA, McKee M. Alteration of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.7. Matsuo T. Basic nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.8. Wei YH. Oxidativestress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998; 217:53–63.9. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007; 84:389–99.10. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123:458–63.11. Brodsky SV, Morrishow AM, Dharia N, et al. Glucose scavenging of nitric oxide. Am J Physiol. 2001; 280:480–6.
Article12. El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003; 44:3135–43.
Article13. Becker B. Diabetes and primary open-angle glaucoma. Am J Ophthalmol. 1971; 1:1–16.14. Davies PD, Duncan G, Pynsent PB, et al. Aqueous humor glucose concentration in cataract patients and its effect on the lens. Exp Eye Res. 1984; 39:605–9.15. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article16. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15 N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.17. Teufelhofer O, Weiss R-M, Parzefall W, et al. Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicol Sci. 2003; 76:376–83.
Article18. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.19. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.20. Haefliger IO, Dettman E, Liu R, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999; 43:S51–8.
Article21. Chakravarthy U, Hayes RG, Stitt AW, et al. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998; 47:945–52.
Article22. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.23. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.
Article24. Brüne B, Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.
Article25. Gross SS, Levi R. Tetrahydrobiopterin synthesis. J Biol Chem. 1992; 267:25722–9.26. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.27. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.28. Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.
Article29. Weinreb RN, Bloom E, Baxter JD, et al. Detection of glucocortocoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci. 1981; 21:403–7.30. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004; 24:413–20.
Article31. Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000; 87:540–2.
Article32. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from ocular tissue: the trabecular meshwork. J Cell Physiol. 1999; 180:182–9.33. Kurz DJ, Decary S, Hong Y, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004; 117:2417–26.
Article34. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000; 908:99–110.
Article35. Furumoto K, Inoue E, Nagao N, et al. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C viasuppression of oxidative stress. Life Sci. 1998; 63:935–48.36. Sato T, Roy S. Effect of high glucose on fibronectin expression and cell proliferation in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43:170–5.37. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987; 1:204–10.
Article38. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.
Article39. Millard CB, Tripathi BJ, Tripathi RC. Age-related changes in protein profiles of the normal human trabecular meshwork. Exp Eye Res. 1987; 45:623–31.
Article40. Schchschabel DO, Binninger E. Aging of trabecular meshwork cells of the human eye in vitro. Z Gerontol. 1990; 23:133–5.41. Horstmann HJ, Rohen JW, Sames K. Age-related changes in the composition of proteins in the trabecular meshwork of the human eye. Mech Ageing Dev. 1983; 21:121–36.
Article42. Park J-Y, Takahara N, Gabriele A, et al. Induction of endothelin-1 expression by glucose. An effect of protein kinase c activation. Diabetes. 2000; 49:1239–48.
Article43. Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003; 114:697–8.
Article44. Wordinger RJ, Clark AF. Effects of glucocortocoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog Ret Eye Res. 1999; 5:629–67.45. Barber AJ, Lieth E, Khin SA, et al. Neuronal apoptosis in the retina during experimental and human diabetes. J Clin Invest. 1998; 102:783–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Effect of Dipyridamole on the Reactive Oxygen Species and Oxidative Stress in Trabecular Meshwork Cells
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- Effect of Ascorbic Acid Against the Oxidative Stress-Induced Cellular Senescence in Trabecular Meshwork Cells
- Cytoprotective Effect of Rho Kinase Inhibitors against Oxidative Stress inTrabecular Meshwork Cells