J Periodontal Implant Sci.
2011 Feb;41(1):17-22. 10.5051/jpis.2011.41.1.17.
Synergic induction of human periodontal ligament fibroblast cell death by nitric oxide and N-methyl-D-aspartic acid receptor antagonist
- Affiliations
-
- 1Department of Life Science, Dongguk University-Seoul, Seoul, Korea.
- 2Department of Cell and Developmental Biology, Seoul National University School of Dentistry, Seoul, Korea.
- 3Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. periopf@snu.ac.kr
- KMID: 2212131
- DOI: http://doi.org/10.5051/jpis.2011.41.1.17
Abstract
- PURPOSE
Nitric oxide (NO) has been known as an important regulator of osteoblasts and periodontal ligament cell activity. This study was performed to investigate the relationship between NO-mediated cell death of human periodontal ligament fibroblasts (PDLFs) and N-methyl-D-aspartic acid (NMDA) receptor antagonist (+)-5-methyl-10, 11-dihydro-5H-dibenzo[a,d]cyclohepten-5, 10-imine hydrogen maleate (MK801).
METHODS
Human PDLFs were treated with various concentrations (0 to 4 mM) of sodium nitroprusside (SNP) with or without 200 microM MK801 in culture media for 16 hours and the cell medium was then removed and replaced by fresh medium containing MTS reagent for cell proliferation assay. Western blot analysis was performed to investigate the effects of SNP on the expression of Bax, cytochrome c, and caspase-3 proteins. The differences for each value among the sample groups were compared using analysis of variance with 95% confidence intervals.
RESULTS
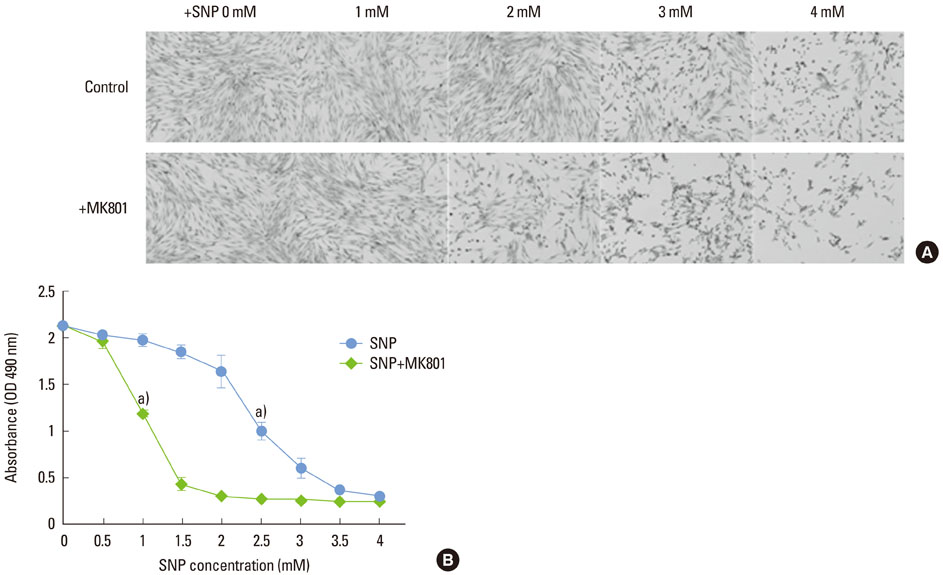
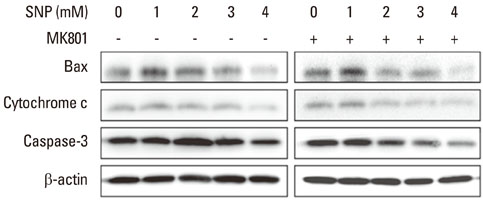
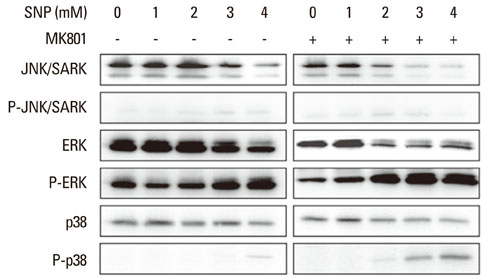
In the case of SNP treatment, as a NO donor, cell viability was significantly decreased in a concentration-dependent manner. In addition, a synergistic effect was shown when both SNP and NMDA receptor antagonist was added to the medium. SNP treated PDLFs exhibited a round shape in culture conditions and were dramatically reduced in cell number. SNP treatment also increased levels of apoptotic marker protein, such as Bax and cytochrome c, and reduced caspase-3 in PDLFs. Mitogen-activated protein kinase signaling was activated by treatment of SNP and NMDA receptor antagonist.
CONCLUSIONS
These results suggest that excessive production of NO may induce apoptosis and that NMDA receptor may modulate NO-induced apoptosis in PDLFs.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Caspase 3
Cell Count
Cell Death
Cell Proliferation
Cell Survival
Culture Media
Cytochromes c
Dizocilpine Maleate
Fibroblasts
Humans
Hydrogen
Maleates
N-Methylaspartate
Nitric Oxide
Nitroprusside
Osteoblasts
Periodontal Ligament
Protein Kinases
Proteins
Tissue Donors
Caspase 3
Culture Media
Cytochromes c
Dizocilpine Maleate
Hydrogen
Maleates
N-Methylaspartate
Nitric Oxide
Nitroprusside
Protein Kinases
Proteins
Figure
Reference
-
1. Chang H, Tsai SY, Chang Y, Chen TL, Chen RM. Therapeutic concentrations of propofol protects mouse macrophages from nitric oxide-induced cell death and apoptosis. Can J Anaesth. 2002; 49:477–480.
Article2. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–142.3. van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001; 103:255–261.4. Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, MacIntyre I. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res Commun. 2000; 274:477–481.
Article5. Koyama A, Otsuka E, Inoue A, Hirose S, Hagiwara H. Nitric oxide accelerates the ascorbic acid-induced osteoblastic differentiation of mouse stromal ST2 cells by stimulating the production of prostaglandin E(2). Eur J Pharmacol. 2000; 391:225–231.
Article6. Ralston SH, Todd D, Helfrich M, Benjamin N, Grabowski PS. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology. 1994; 135:330–336.
Article7. Damoulis PD, Hauschka PV. Cytokines induce nitric oxide production in mouse osteoblasts. Biochem Biophys Res Commun. 1994; 201:924–931.
Article8. Armour KE, Van'T Hof RJ, Grabowski PS, Reid DM, Ralston SH. Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. J Bone Miner Res. 1999; 14:2137–2142.
Article9. Brunetti L. Nitric oxide: a gas as a modulator of neuroendocrine secretions. Clin Ter. 1994; 144:147–153.10. Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000; 32:476–509.
Article11. Arundine M, Sanelli T, Ping He B, Strong MJ. NMDA induces NOS 1 translocation to the cell membrane in NGF-differentiated PC 12 cells. Brain Res. 2003; 976:149–158.
Article12. Hinoi E, Fujimori S, Yoneda Y. Modulation of cellular differentiation by N-methyl-D-aspartate receptors in osteoblasts. FASEB J. 2003; 17:1532–1534.13. Taylor AF. Osteoblastic glutamate receptor function regulates bone formation and resorption. J Musculoskelet Neuronal Interact. 2002; 2:285–290.14. Yu JH, Lee SP, Kim TI, Jang JH. Identification of N-methyl-D-aspartate receptor subunit in human periodontal ligament fibroblasts: potential role in regulating differentiation. J Periodontol. 2009; 80:338–346.
Article15. Tomokiyo A, Maeda H, Fujii S, Wada N, Shima K, Akamine A. Development of a multipotent clonal human periodontal ligament cell line. Differentiation. 2008; 76:337–347.
Article16. Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod. 1997; 19:615–621.
Article17. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article18. Kikuiri T, Hasegawa T, Yoshimura Y, Shirakawa T, Oguchi H. Cyclic tension force activates nitric oxide production in cultured human periodontal ligament cells. J Periodontol. 2000; 71:533–539.
Article19. Watarai H, Warita H, Soma K. Effect of nitric oxide on the recovery of the hypofunctional periodontal ligament. J Dent Res. 2004; 83:338–342.
Article20. Armour KE, Ralston SH. Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology. 1998; 139:799–802.
Article21. Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, et al. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun. 1998; 250:108–114.
Article22. Chen RM, Chen TL, Chiu WT, Chang CC. Molecular mechanism of nitric oxide-induced osteoblast apoptosis. J Orthop Res. 2005; 23:462–468.
Article23. Palmer RM, Bridge L, Foxwell NA, Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol. 1992; 105:11–12.
Article24. Lin SK, Kok SH, Lin LD, Wang CC, Kuo MY, Lin CT, et al. Nitric oxide promotes the progression of periapical lesion via inducing macrophage and osteoblast apoptosis. Oral Microbiol Immunol. 2007; 22:24–29.
Article25. Thammasitboon K, Goldring SR, Boch JA. Role of macrophages in LPS-induced osteoblast and PDL cell apoptosis. Bone. 2006; 38:845–852.
Article26. Alexander MB, Damoulis PD. The role of cytokines in the pathogenesis of periodontal disease. Curr Opin Periodontol. 1994; 39–53.27. Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007; 78:550–558.
Article28. Reddi K, Meghji S, Wilson M, Henderson B. Comparison of the osteolytic activity of surface-associated proteins of bacteria implicated in periodontal disease. Oral Dis. 1995; 1:26–31.
Article29. Sosroseno W, Bird PS, Seymour GJ. Nitric oxide production by a human osteoblast cell line stimulated with Aggregatibacter actinomycetemcomitans lipopolysaccharide. Oral Microbiol Immunol. 2009; 24:50–55.
Article30. Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993; 150:5080–5085.31. Tiwari MM, Messer KJ, Mayeux PR. Inducible nitric oxide synthase and apoptosis in murine proximal tubule epithelial cells. Toxicol Sci. 2006; 91:493–500.
Article32. Xie K, Huang S, Dong Z, Juang SH, Wang Y, Fidler IJ. Destruction of bystander cells by tumor cells transfected with inducible nitric oxide (NO) synthase gene. J Natl Cancer Inst. 1997; 89:421–427.
Article33. Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999; 1411:217–230.
Article34. Chen CC, Kanno Z, Soma K. Occlusal forces promote periodontal healing of transplanted teeth with enhanced nitric oxide synthesis. J Med Dent Sci. 2005; 52:59–64.35. Chen RM, Liu HC, Lin YL, Jean WC, Chen JS, Wang JH. Nitric oxide induces osteoblast apoptosis through the de novo synthesis of Bax protein. J Orthop Res. 2002; 20:295–302.
Article36. Srivastava RK, Sollott SJ, Khan L, Hansford R, Lakatta EG, Longo DL. Bcl-2 and Bcl-X(L) block thapsigargin-induced nitric oxide generation, c-Jun NH(2)-terminal kinase activity, and apoptosis. Mol Cell Biol. 1999; 19:5659–5674.
Article37. Ho WP, Chen TL, Chiu WT, Tai YT, Chen RM. Nitric oxide induces osteoblast apoptosis through a mitochondria-dependent pathway. Ann N Y Acad Sci. 2005; 1042:460–470.
Article38. Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999; 68:383–424.
Article39. Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001; 26:390–397.
Article40. Brüne B, von Knethen A, Sandau KB. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999; 6:969–975.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of nitric oxide on the proliferation and differentiation of human periodontal ligament cells
- Inhibition of lyosphosphatidic acid receptor 1 signaling in periodontal ligament stem cells reduces inflammatory paracrine effect in primary astrocyte cells
- Effect of Nitric Oxide on the Proliferation of Cultured Human Tenon Capsule Fibroblasts Exposed to Benzalkonium Chloride
- Role of Nitric Oxide on the Proliferation of Human Tenon Capsule Fibroblasts
- Identification of Differentially Expressed Genes in Murine Hippocampus by Modulation of Nitric Oxide in Kainic Acid-induced Neurotoxic Animal Model