J Korean Rheum Assoc.
2009 Mar;16(1):23-32. 10.4078/jkra.2009.16.1.23.
The Marginal Zone B Cells have an Increased Antibody Expression in Mice with Collagen-induced Arthritis
- Affiliations
-
- 1The Rheumatism Research Center, Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea. rmin6403@hanmail.net
- KMID: 2202137
- DOI: http://doi.org/10.4078/jkra.2009.16.1.23
Abstract
OBJECTIVE
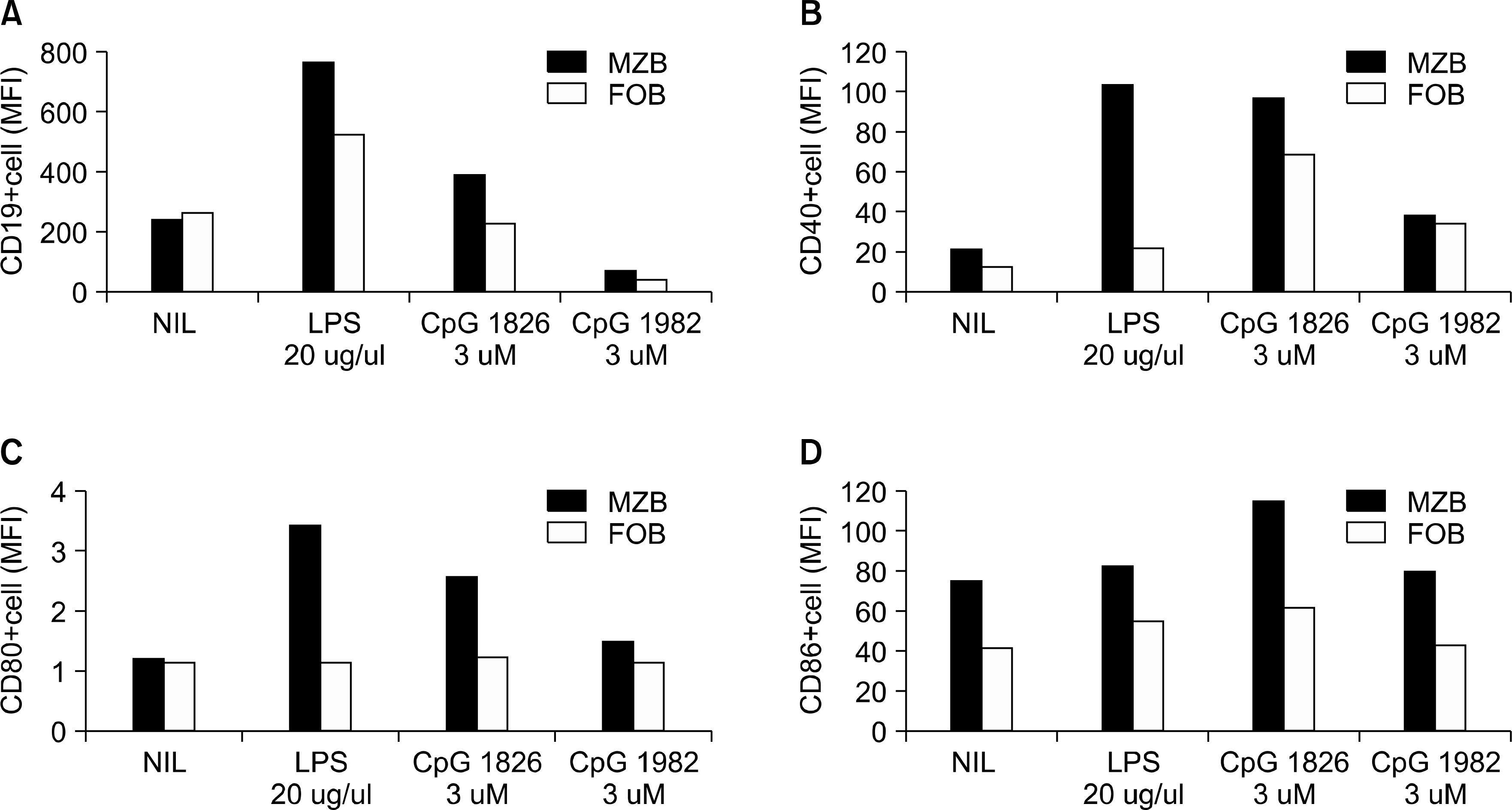
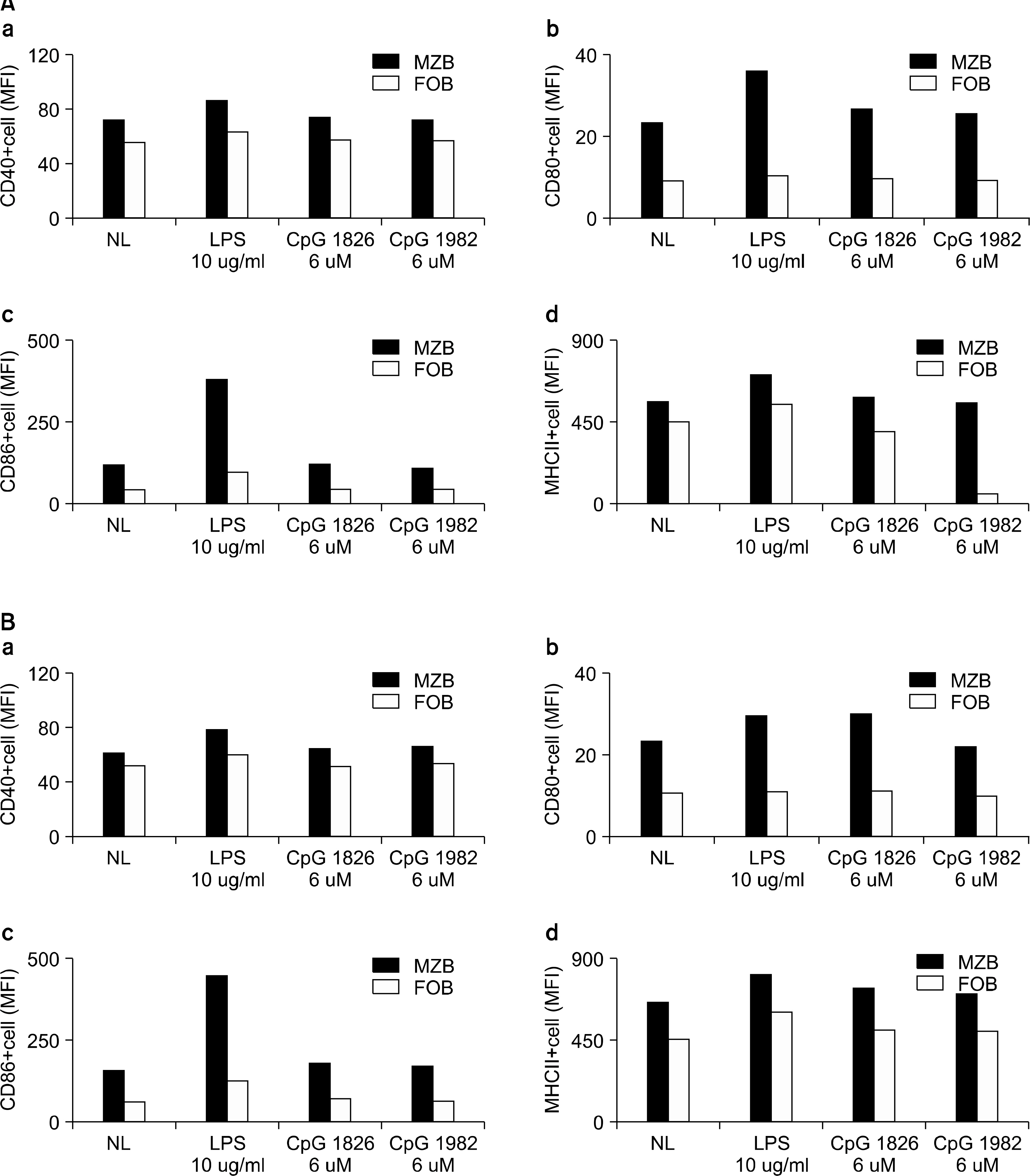
Mature B cells in the spleen of mouse can be divide into two main subsets: the follicular (FO) B cells and the marginal zone (MZ) B cells. In this study, we investigated which subtype of B cells is involved in the production of costimulatory molecules, cytokine and antibody during the induction of autoimmune arthritis.
METHODS
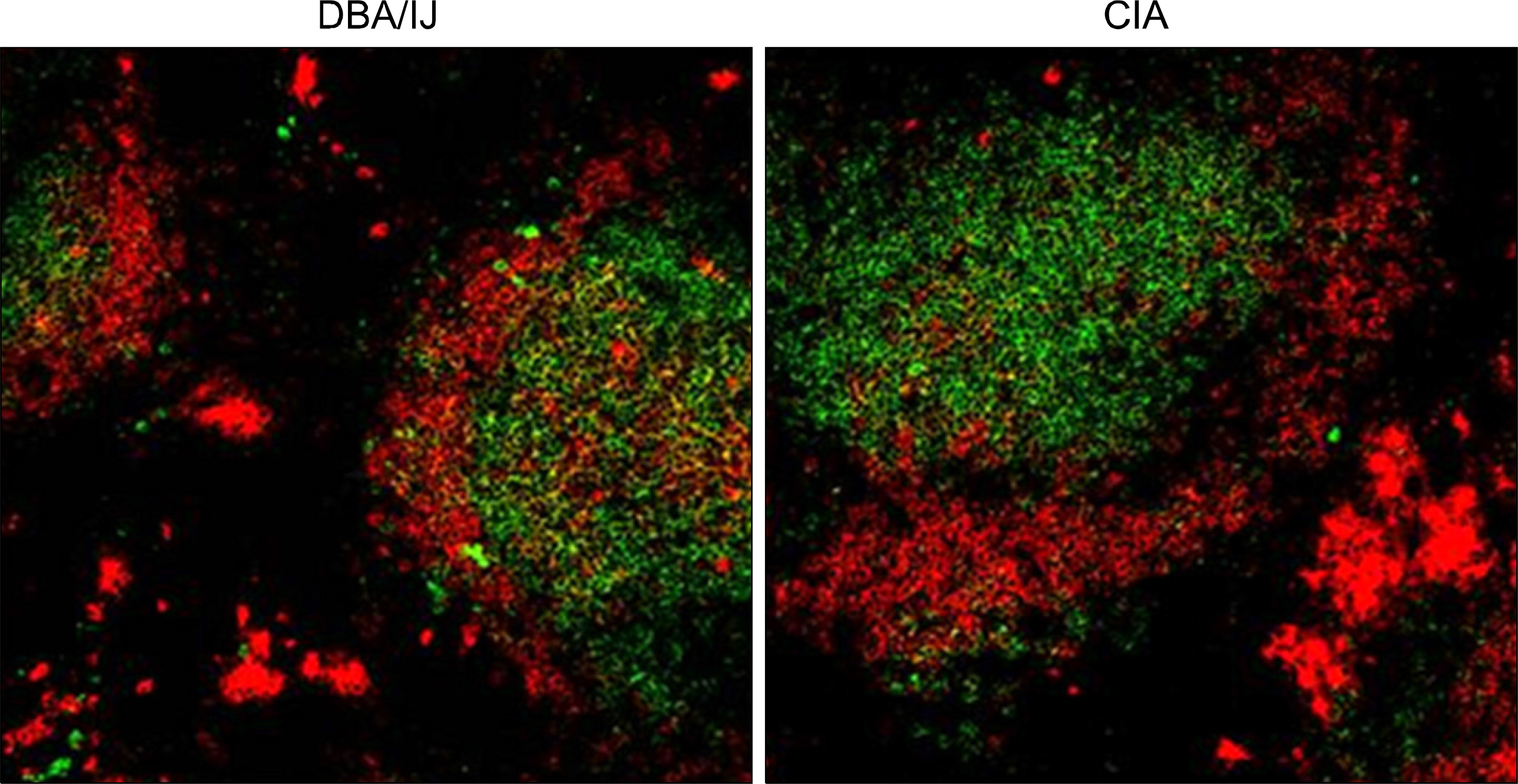
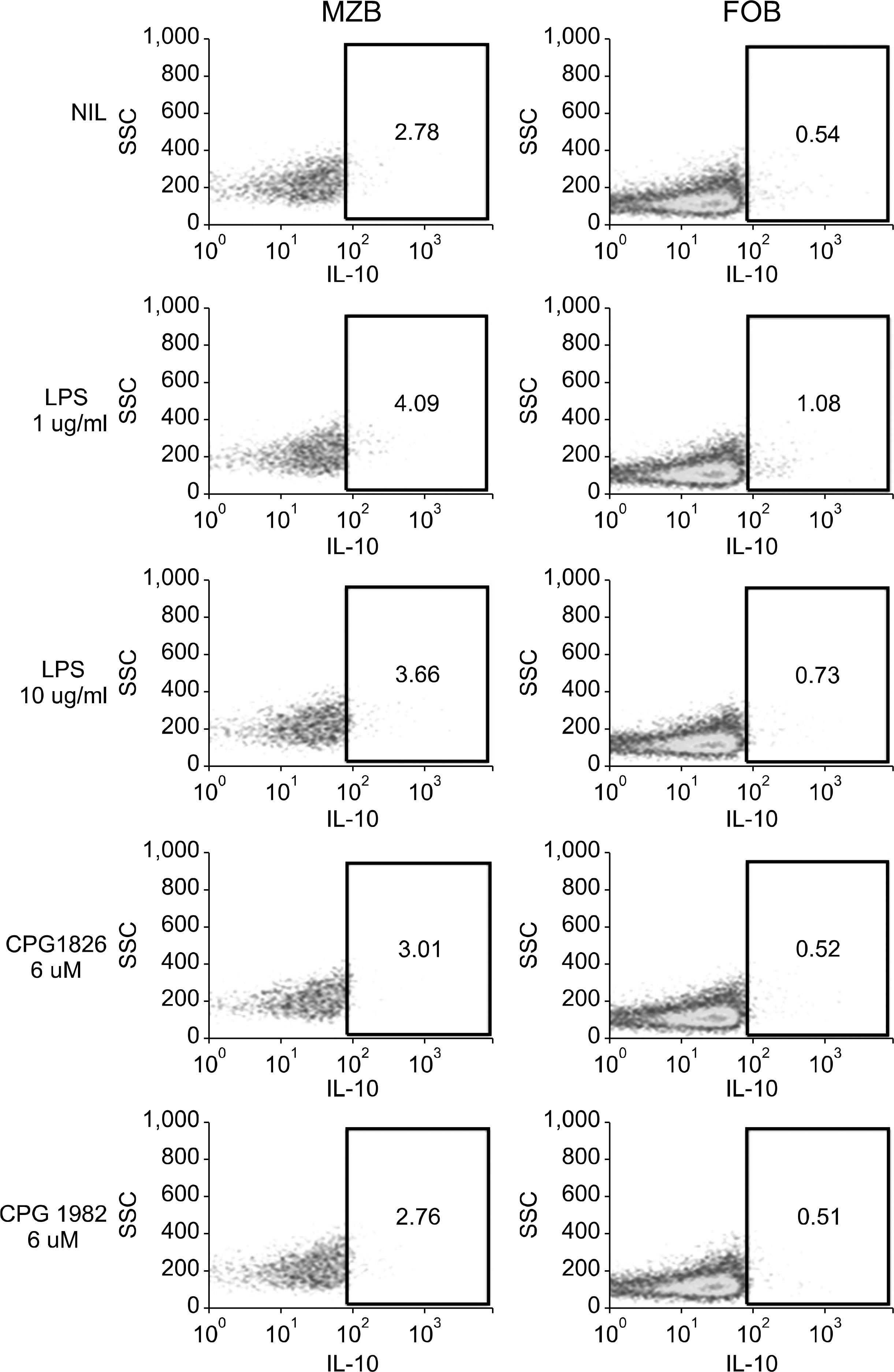
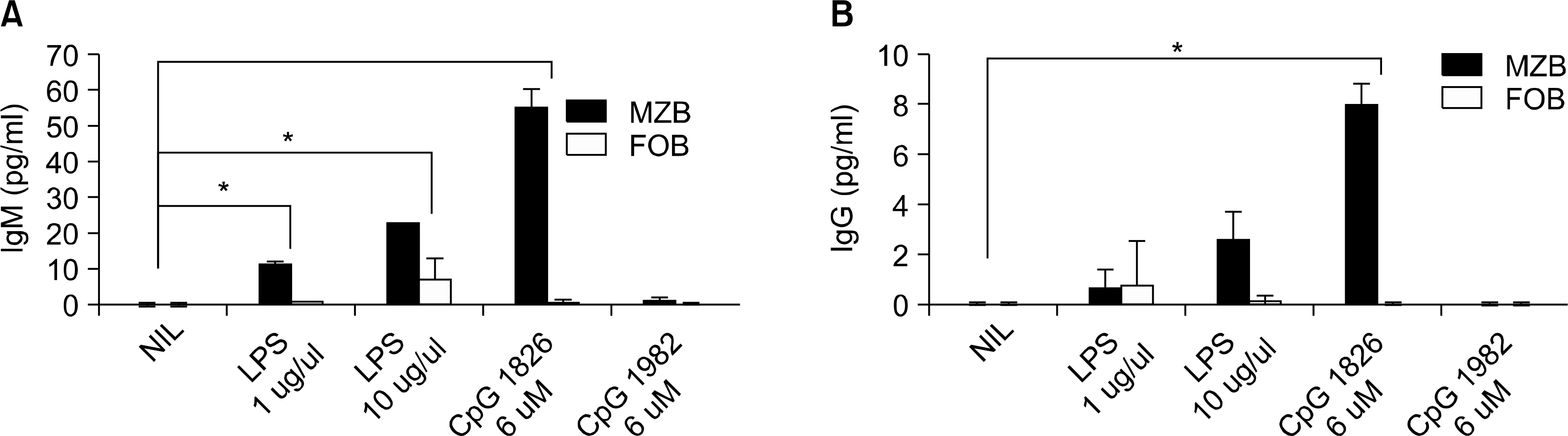
The MZB and FOB cells isolated from DBA/1J induced- and collagen-induced arthritis (CIA) mice were stimulated with LPS or CpG. The costimulatory molecules were measured by flow cytometry (FACs). The cytokines were measured by ELISA. Production of antibodies by the MZB cells or FOB cells was measured by ELISA and the results were observed by confocal microscopy.
RESULTS
The expression of co-stimulatory molecules was stronger in the MZB cells than that in the FOB cells. The production of cytokines (IL-10, IL-6) and antibodies was higher in the MZB cells. The IgG expression of the MZB cells, which is known to be associated with the acceleration of autoimmunity, was higher in the CIA mice than that in the DBA/1J mice.
CONCLUSION
We observed that the MZB cells were increased in the CIA mice. The costimulatory molecules, cytokine and auto-antibodies were increased in the MZB cells compared to that of the FOB cells. Our results suggest that MZB cells mainly produce autoantibodies, and they play a key role in development of autoimmune arthritis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wang Y, Zhang P, Li W, Hou L, Wang J, Liang Y, et al. Mouse follicular and marginal zone B cells show differential expression of Dnmt3a and sensitivity to 5'-azacytidine. Immunol Lett. 2006; 105:174–9.
Article2. Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997; 27:2366–74.
Article3. Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a "natural immune memory". Immunol Rev. 2000; 175:70–9.4. Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002; 2:323–35.
Article5. Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005; 23:161–96.
Article6. McHeyzer-Williams MG. B cells as effectors. Curr Opin Immunol. 2003; 15:354–61.
Article7. Gunn KE, Brewer JW. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J Immunol. 2006; 177:3791–8.
Article8. Cinamon G, Zachariah MA, Lam OM, Foss FW Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008; 9:54–62.
Article9. Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999; 162:7198–207.10. Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001; 167:1886–90.
Article11. Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebocontrolled trial. Arthritis Rheum. 1998; 41:290–7.
Article12. Srivastava B, Lindsley RC, Nikbakht N, Allman D. Models for peripheral B cell development and homeostasis. Semin Immunol. 2005; 17:175–82.
Article13. Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000; 164:5000–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fluorescence bioimaging analysis of collagen antibody-induced arthritis in male mice
- Effect of a Flavon Extracted from Artemisia absinthium on Collagen Induced Arthritis in Mice
- Collagen-Induced Arthritis and the Potential Role of Autoimmunity to Type II Collagen in Rheumatoid Arthritis
- The Role of Lipocortin-1 in the Corticosteroid Action in Collagen Induced Arthritis
- The Expression of Toll-like Receptors in Collagen-induced Arthritis