J Korean Neurosurg Soc.
2014 Jun;55(6):307-312. 10.3340/jkns.2014.55.6.307.
Semi-Quantitative Analyses of Hippocampal Heat Shock Protein-70 Expression Based on the Duration of Ischemia and the Volume of Cerebral Infarction in Mice
- Affiliations
-
- 1Department of Neurosurgery, Ansan Hospital, Korea University Medical Center, Korea University College of Medicine, Ansan, Korea. sungkha@yahoo.com
- KMID: 2191109
- DOI: http://doi.org/10.3340/jkns.2014.55.6.307
Abstract
OBJECTIVE
We investigated the expression of hippocampal heat shock protein 70 (HSP-70) infarction volume after different durations of experimental ischemic stroke in mice.
METHODS
Focal cerebral ischemia was induced in mice by occluding the middle cerebral artery with the modified intraluminal filament technique. Twenty-four hours after ischemia induction, both hippocampi were extracted for HSP-70 protein analyses. Slices from each hemisphere were stained with 2,3,5-triphenyltetrazolium chloride (2%), and infarction volumes were calculated. HSP-70 levels were evaluated using western blot and enzyme-linked immunosorbent assay (ELISA). HSP-70 subtype (hsp70.1, hspa1a, hspa1b) mRNA levels in the hippocampus were measured using reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS
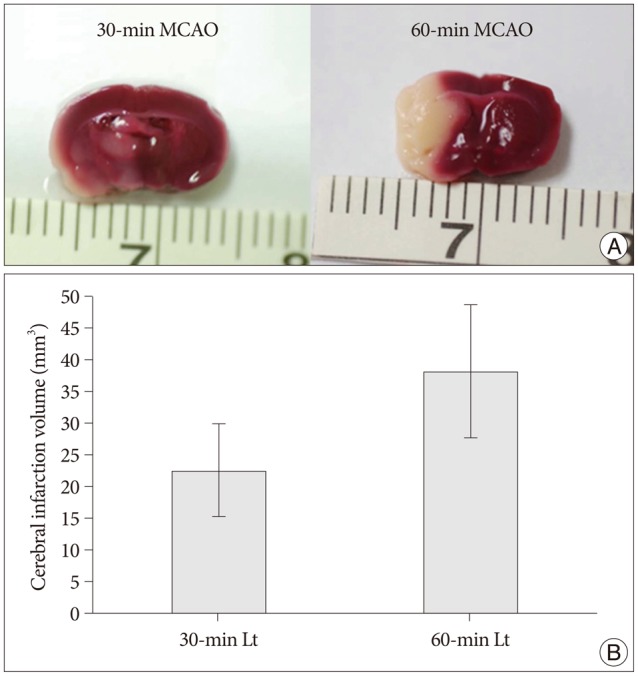
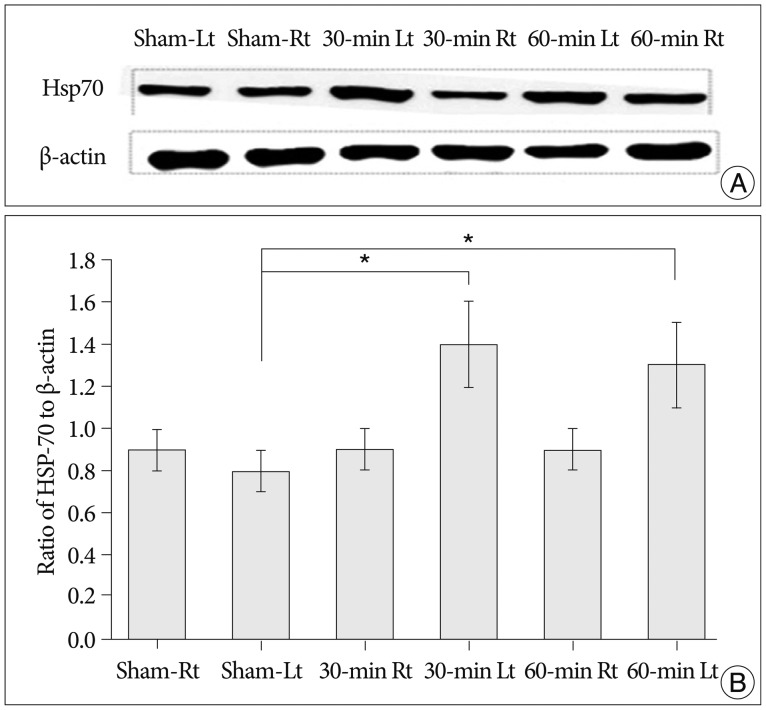
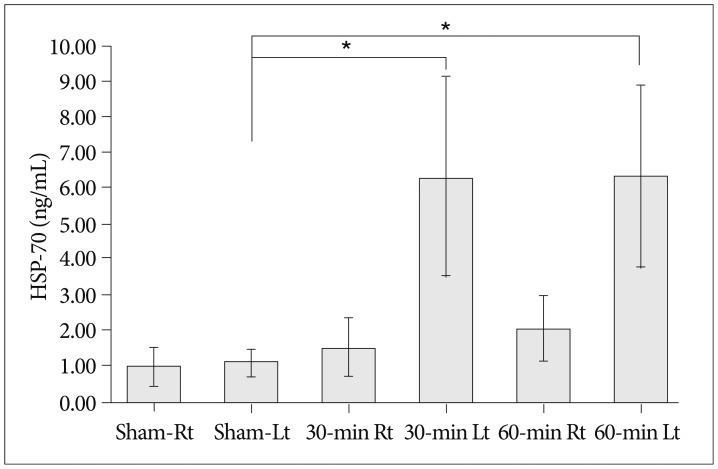
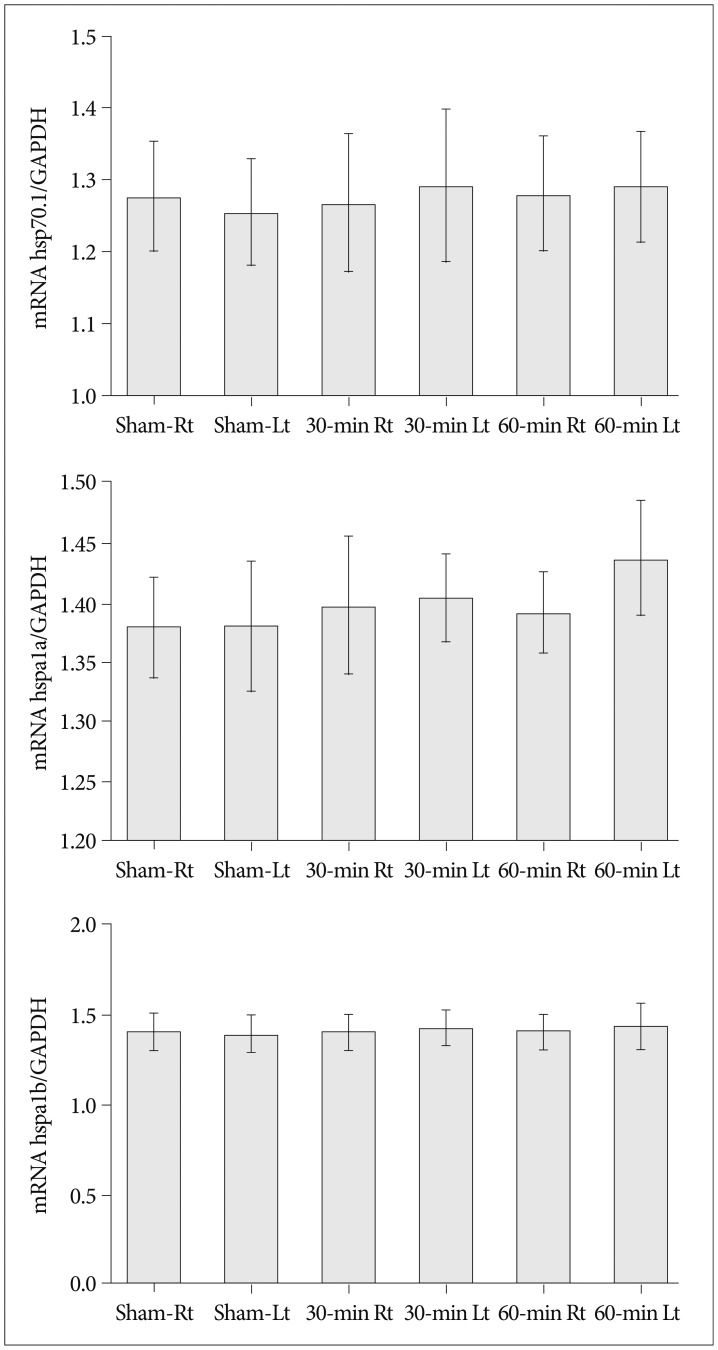
Cerebral infarctions were found ipsilateral to the occlusion in 10 mice exposed to transient ischemia (5 each in the 30-min and 60-min occlusion groups), whereas no focal infarctions were noted in any of the sham mice. The average infarct volumes of the 2 ischemic groups were 22.28+/-7.31 mm3 [30-min group+/-standard deviation (SD)] and 38.06+/-9.53 mm3 (60-min group+/-SD). Western blot analyses and ELISA showed that HSP-70 in hippocampal tissues increased in the infarction groups than in the sham group. However, differences in HSP-70 levels between the 2 infarction groups were statistically insignificant. Moreover, RT-PCR results demonstrated no relationship between the mRNA expression of HSP-70 subtypes and occlusion time or infarction volume.
CONCLUSION
Our results indicated no significant difference in HSP-70 expression between the 30- and 60-min occlusion groups despite the statistical difference in infarction volumes. Furthermore, HSP-70 subtype mRNA expression was independent of both occlusion duration and cerebral infarction volume.
MeSH Terms
Figure
Reference
-
1. Abe K, Kawagoe J, Araki T, Aoki M, Kogure K. Differential expression of heat shock protein 70 gene between the cortex and caudate after transient focal cerebral ischaemia in rats. Neurol Res. 1992; 14:381–385. PMID: 1362251.
Article2. Akai F, Yanagihara T. Identity of the dorsal hippocampal region most vulnerable to cerebral ischemia. Brain Res. 1993; 603:87–95. PMID: 8453479.
Article3. Blagosklonny MV. Re : Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2001; 93:239–240. PMID: 11158196.
Article4. Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007; 1113:147–158. PMID: 17656567.
Article5. Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004; 16:53–61. PMID: 14676570.
Article6. Hata R, Mies G, Wiessner C, Hossmann KA. Differential expression of c-fos and hsp72 mRNA in focal cerebral ischemia of mice. Neuroreport. 1998; 9:27–32. PMID: 9592042.
Article7. Lee KJ, Terada K, Oyadomari S, Inomata Y, Mori M, Gotoh T. Induction of molecular chaperones in carbon tetrachloride-treated rat liver : implications in protection against liver damage. Cell Stress Chaperones. 2004; 9:58–68. PMID: 15270078.
Article8. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20:84–91. PMID: 2643202.
Article9. Massa SM, Swanson RA, Sharp FR. The stress gene response in brain. Cerebrovasc Brain Metab Rev. 1996; 8:95–158. PMID: 8727184.10. Melnikova VO, Balasubramanian K, Villares GJ, Dobroff AS, Zigler M, Wang H, et al. Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. J Biol Chem. 2009; 284:28845–28855. PMID: 19703903.
Article11. Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response : regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997; 32:17–29. PMID: 9493008.12. Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989; 20:1037–1043. PMID: 2756535.
Article13. Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003; 304:505–512. PMID: 12729585.
Article14. Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood). 2003; 228:111–133. PMID: 12563018.
Article15. Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000; 47:782–791. PMID: 10852544.
Article16. Roberts GG, Di Loreto MJ, Marshall M, Wang J, DeGracia DJ. Hippocampal cellular stress responses after global brain ischemia and reperfusion. Antioxid Redox Signal. 2007; 9:2265–2275. PMID: 17715997.
Article17. Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto R, et al. DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res. 2002; 108:81–93. PMID: 12480181.
Article18. Snider BJ, Du C, Wei L, Choi DW. Cycloheximide reduces infarct volume when administered up to 6 h after mild focal ischemia in rats. Brain Res. 2001; 917:147–157. PMID: 11640900.
Article19. Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990; 10:290–293. PMID: 1689322.
Article20. van der Weerd L, Lythgoe MF, Badin RA, Valentim LM, Akbar MT, de Belleroche JS, et al. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp Neurol. 2005; 195:257–266. PMID: 15936758.
Article21. Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008; 68:9078–9086. PMID: 18974154.
Article22. Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007; 594:89–99. PMID: 17205678.
Article23. Yaglom JA, Ekhterae D, Gabai VL, Sherman MY. Regulation of necrosis of H9c2 myogenic cells upon transient energy deprivation. Rapid deenergization of mitochondria precedes necrosis and is controlled by reactive oxygen species, stress kinase JNK, HSP72 and ARC. J Biol Chem. 2003; 278:50483–50496. PMID: 14523009.
Article24. Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002; 513:281–299. PMID: 12575825.
Article25. Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70). Mol Med Today. 1999; 5:525–531. PMID: 10562718.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heat-Shock Protein 70 Attenuates Caspase-3 Activation and DNA Fragmentation Following Focal Cerebral Ischemia
- The Significance of Induction of Heat Shock Protein-70 and Glial Fibrillary Acidic Protein Messenger RNA by Delayed Postischemic Hyperthermia Following Transient Focal Ischemia
- S100ß, Matrix Metalloproteinase-9, D-dimer, and Heat Shock Protein 70 Are Serologic Biomarkers of Acute Cerebral Infarction in a Mouse Model of Transient MCA Occlusion
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein