J Korean Neurosurg Soc.
2013 Dec;54(6):467-476. 10.3340/jkns.2013.54.6.467.
Engraftment of Human Mesenchymal Stem Cells in a Rat Photothrombotic Cerebral Infarction Model : Comparison of Intra-Arterial and Intravenous Infusion Using MRI and Histological Analysis
- Affiliations
-
- 1Department of Radiology, Chung-Ang University College of Medicine, Seoul, Korea. kwakbk@cau.ac.kr
- KMID: 2190941
- DOI: http://doi.org/10.3340/jkns.2013.54.6.467
Abstract
OBJECTIVE
This study aimed to evaluate the hypotheses that administration routes [intra-arterial (IA) vs. intravenous (IV)] affect the early stage migration of transplanted human bone marrow-derived mesenchymal stem cells (hBM-MSCs) in acute brain infarction.
METHODS
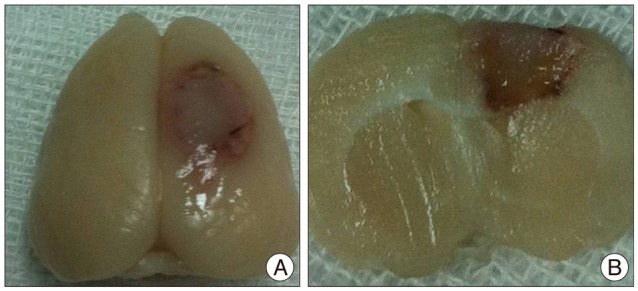
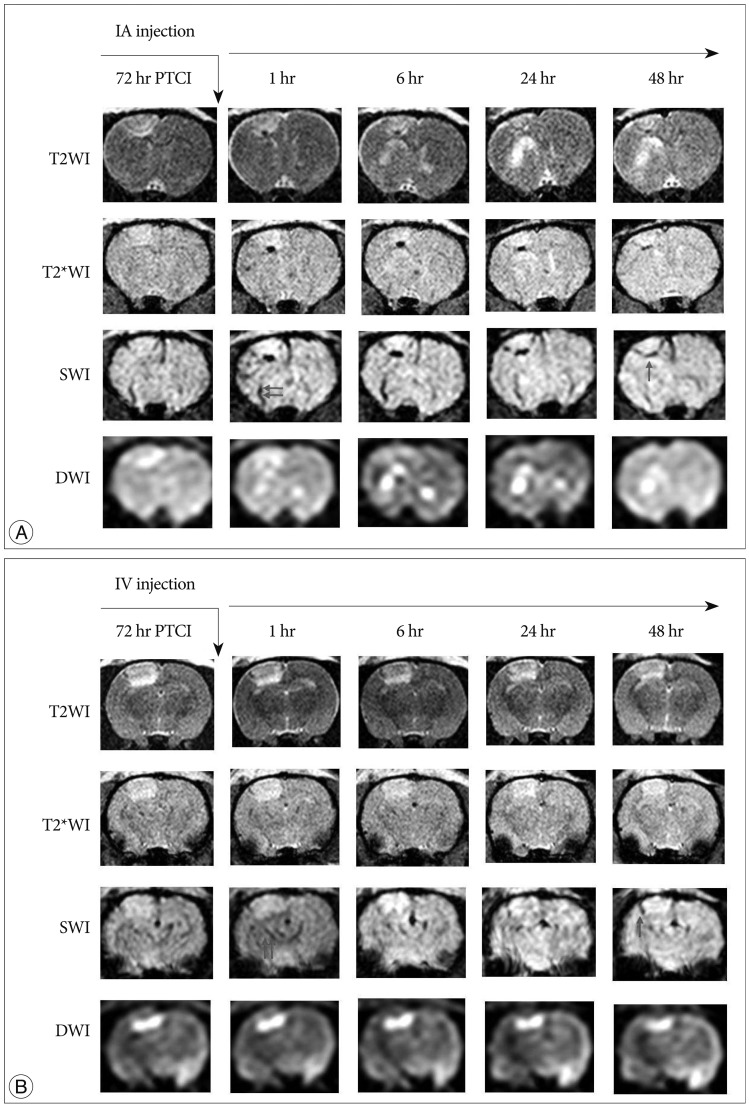
Male Sprague-Dawley rats (n=40) were subjected to photothrombotic infarction. Three days after photothrombotic infarction, rats were randomly allocated to one of four experimental groups [IA group : n=12, IV group : n=12, superparamagnetic iron oxide (SPIO) group : n=8, control group : n=8]. All groups were subdivided into 1, 6, 24, and 48 hours groups according to time point of sacrifice. Magnetic resonance imaging (MRI) consisting of T2 weighted image (T2WI), T2* weighted image (T2*WI), susceptibility weighted image (SWI), and diffusion weighted image of rat brain were obtained prior to and at 1, 6, 24, and 48 hours post-implantation. After final MRI, rats were sacrificed and grafted cells were analyzed in brain and lung specimen using Prussian blue and immunohistochemical staining.
RESULTS
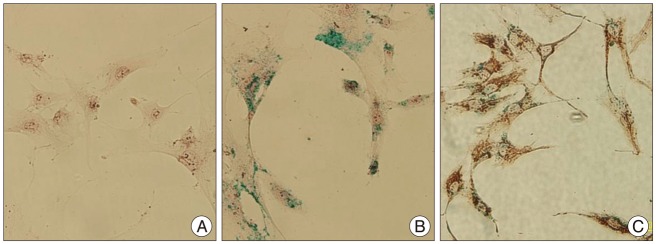
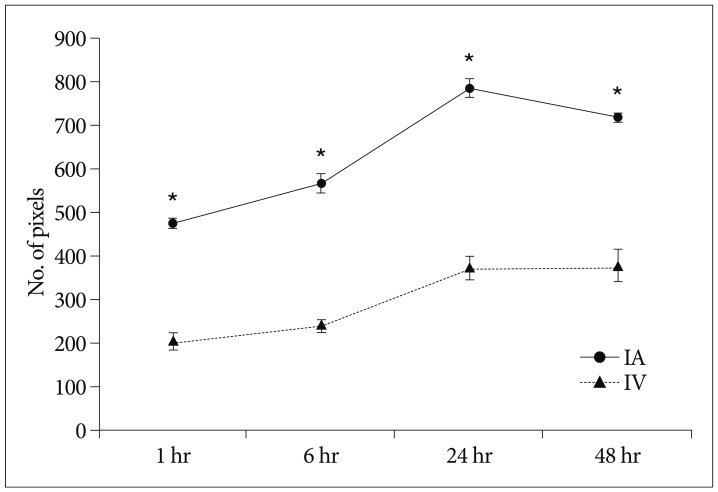
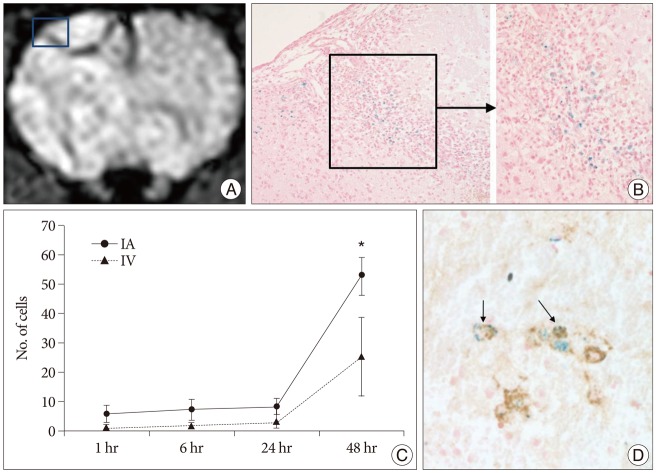
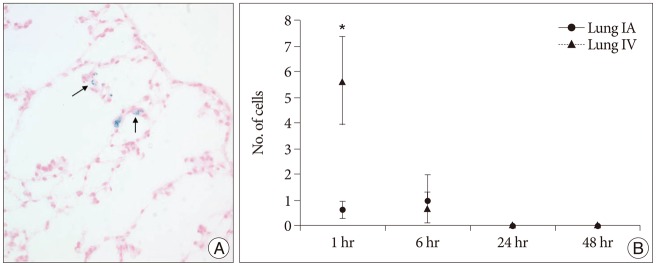
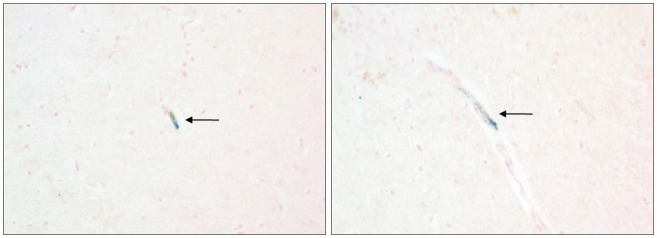
Grafted cells appeared as dark signal intensity regions at the peri-lesional zone. In IA group, dark signals in peri-lesional zone were more prominent compared with IV group. SWI showed largest dark signal followed by T2*WI and T2WI in both IA and IV groups. On Prussian blue staining, IA administration showed substantially increased migration and a large number of transplanted hBM-MSCs in the target brain than IV administration. The Prussian blue-positive cells were not detected in SPIO and control groups.
CONCLUSION
In a rat photothrombotic model of ischemic stroke, selective IA administration of human mesenchymal stem cells is more effective than IV administration. MRI and histological analyses revealed the time course of cell migration, and the numbers and distribution of hBM-MSCs delivered into the brain.
Keyword
MeSH Terms
Figure
Reference
-
1. Abdallah BM, Haack-Sørensen M, Burns JS, Elsnab B, Jakob F, Hokland P, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005; 326:527–538. PMID: 15596132.
Article2. Akter M, Hirai T, Hiai Y, Kitajima M, Komi M, Murakami R, et al. Detection of hemorrhagic hypointense foci in the brain on susceptibility-weighted imaging clinical and phantom studies. Acad Radiol. 2007; 14:1011–1019. PMID: 17707307.
Article3. Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK, et al. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011; 42:2923–2931. PMID: 21836091.
Article4. Andres RH, Choi R, Steinberg GK, Guzman R. Potential of adult neural stem cells in stroke therapy. Regen Med. 2008; 3:893–905. PMID: 18947311.
Article5. Arai T, Kofidis T, Bulte JW, de Bruin J, Venook RD, Berry GJ, et al. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn Reson Med. 2006; 55:203–209. PMID: 16315206.
Article6. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005; 57:874–882. PMID: 15929052.
Article7. Barry FP, Murphy JM. Mesenchymal stem cells : clinical applications and biological characterization. Int J Biochem Cell Biol. 2004; 36:568–584. PMID: 15010324.
Article8. Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004; 1010:108–116. PMID: 15126123.
Article9. Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, et al. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res. 2004; 1009:26–33. PMID: 15120580.
Article10. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain : expression of neuronal phenotypes in adult mice. Science. 2000; 290:1775–1779. PMID: 11099418.
Article11. Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke. 1996; 27:1105–1109. discussion 1109-1111. PMID: 8650722.
Article12. Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004; 5:567–584. PMID: 15579045.
Article13. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003; 73:778–786. PMID: 12949903.
Article14. Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001; 189:49–57. PMID: 11535233.
Article15. Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003; 92:692–699. PMID: 12609969.
Article16. Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002; 22:275–279. PMID: 12564767.
Article17. Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002; 1:92–100. PMID: 12849513.
Article18. Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004; 1016:145–153. PMID: 15246850.
Article19. Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997; 19:641–648. PMID: 9427967.
Article20. Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007; 25:2777–2785. PMID: 17641243.
Article21. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004; 113:1701–1710. PMID: 15199405.
Article22. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery : the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692. PMID: 19099374.
Article23. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970; 3:393–403. PMID: 5523063.
Article24. Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002; 33:2115–2122. PMID: 12154274.
Article25. Garot J, Unterseeh T, Teiger E, Champagne S, Chazaud B, Gherardi R, et al. Magnetic resonance imaging of targeted catheter-based implantation of myogenic precursor cells into infarcted left ventricular myocardium. J Am Coll Cardiol. 2003; 41:1841–1846. PMID: 12767674.
Article26. Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK. Intravascular cell replacement therapy for stroke. Neurosurg Focus. 2008; 24:E15. PMID: 18341391.
Article27. Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008; 39:1300–1306. PMID: 18309158.
Article28. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004; 52:612–618. PMID: 15334582.
Article29. Hicks A, Jolkkonen J. Challenges and possibilities of intravascular cell therapy in stroke. Acta Neurobiol Exp (Wars). 2009; 69:1–11. PMID: 19325636.30. Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003; 108:1009–1014. PMID: 12912822.
Article31. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011; 134(Pt 6):1790–1807. PMID: 21493695.
Article32. Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005; 18:366–374. PMID: 15686965.
Article33. Kassem M. Mesenchymal stem cells : biological characteristics and potential clinical applications. Cloning Stem Cells. 2004; 6:369–374. PMID: 15671665.
Article34. Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice : evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998; 18:570–579. PMID: 9591849.
Article35. Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, et al. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007; 67:620–625. discussion 625. PMID: 17512331.
Article36. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010; 28:1099–1106. PMID: 20506226.
Article37. Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006; 114(1 Suppl):I94–I100. PMID: 16820652.
Article38. Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010; 30:653–662. PMID: 19888287.
Article39. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat : neurotrophins and functional recovery. Neurology. 2002; 59:514–523. PMID: 12196642.
Article40. Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005; 49:407–417. PMID: 15540231.
Article41. Liu W, Jiang X, Fu X, Cui S, Du M, Cai Y, et al. Bone marrow stromal cells can be delivered to the site of traumatic brain injury via intrathecal transplantation in rabbits. Neurosci Lett. 2008; 434:160–164. PMID: 18325665.
Article42. Lundberg J, Le Blanc K, Söderman M, Andersson T, Holmin S. Endovascular transplantation of stem cells to the injured rat CNS. Neuroradiology. 2009; 51:661–667. PMID: 19562330.
Article43. Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971; 11:345–349. PMID: 5136066.
Article44. Magnitsky S, Watson DJ, Walton RM, Pickup S, Bulte JW, Wolfe JH, et al. In vivo and ex vivo MRI detection of localized and disseminated neural stem cell grafts in the mouse brain. Neuroimage. 2005; 26:744–754. PMID: 15955483.
Article45. McAuley MA. Rodent models of focal ischemia. Cerebrovasc Brain Metab Rev. 1995; 7:153–180. PMID: 7669493.46. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain : cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000; 290:1779–1782. PMID: 11099419.
Article47. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007; 40:609–619. PMID: 17603514.
Article48. Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010; 41:2064–2070. PMID: 20616329.
Article49. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005; 436:266–271. PMID: 16015332.
Article50. Rebenko-Moll NM, Liu L, Cardona A, Ransohoff RM. Chemokines, mononuclear cells and the nervous system : heaven (or hell) is in the details. Curr Opin Immunol. 2006; 18:683–689. PMID: 17010588.
Article51. Reddy AM, Kwak BK, Shim HJ, Ahn C, Cho SH, Kim BJ, et al. Functional characterization of mesenchymal stem cells labeled with a novel PVP-coated superparamagnetic iron oxide. Contrast Media Mol Imaging. 2009; 4:118–126. PMID: 19308999.
Article52. Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain : MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997; 204:272–277. PMID: 9205259.
Article53. Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005; 22:439–450. PMID: 16163700.
Article54. Sehgal V, Delproposto Z, Haddar D, Haacke EM, Sloan AE, Zamorano LJ, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging. 2006; 24:41–51. PMID: 16755540.
Article55. Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007; 27:6–13. PMID: 16596121.
Article56. Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006; 137:393–399. PMID: 16298076.
Article57. Shichinohe H, Kuroda S, Yano S, Ohnishi T, Tamagami H, Hida K, et al. Improved expression of gamma-aminobutyric acid receptor in mice with cerebral infarct and transplanted bone marrow stromal cells : an autoradiographic and histologic analysis. J Nucl Med. 2006; 47:486–491. PMID: 16513618.58. Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004; 76:20–34. PMID: 15048927.
Article59. Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008; 39:1569–1574. PMID: 18323495.
Article60. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007; 184:53–68. PMID: 17188755.
Article61. Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008; 1195:104–112. PMID: 18206136.
Article62. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985; 17:497–504. PMID: 4004172.
Article63. Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells : switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005; 23:392–402. PMID: 15749934.
Article64. Witte OW, Stoll G. Delayed and remote effects of focal cortical infarctions : secondary damage and reactive plasticity. Adv Neurol. 1997; 73:207–227. PMID: 8959216.65. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. PMID: 10931522.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Engraftment of Superparamagnetic Iron Oxide-Labeled Mesenchymal Stem Cells Using Three-Dimensional Reconstruction of Magnetic Resonance Imaging in Photothrombotic Cerebral Infarction Models of Rats
- Neurogenin-1 Overexpression Increases the Therapeutic Effects of Mesenchymal Stem Cells through Enhanced Engraftment in an Ischemic Rat Brain
- A therapeutic study of stem cell transplantation in rat stroke model
- The Effect of Combined Therapy of Exercise and Nootropic Agent on Cognitive Function in Focal Cerebral Infarction Rat Model
- Comparison of the Outcomes after Intralesional, Intracisternal, and Intravenous Transplantation of Human Bone Marrow Derived Mesenchymal Stem Cells for Spinal Cord Injured Rat