J Korean Neurosurg Soc.
2013 Jun;53(6):323-330. 10.3340/jkns.2013.53.6.323.
A Minimally Invasive Rabbit Model of Progressive and Reproducible Disc Degeneration Confirmed by Radiology, Gene Expression, and Histology
- Affiliations
-
- 1Department of Neurosurgery, Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea. neuriac@skku.edu
- KMID: 2190818
- DOI: http://doi.org/10.3340/jkns.2013.53.6.323
Abstract
OBJECTIVE
To develop a simple, reproducible model of disc degeneration in rabbits through percutaneous annular puncture and to confirm the degree of degeneration over time.
METHODS
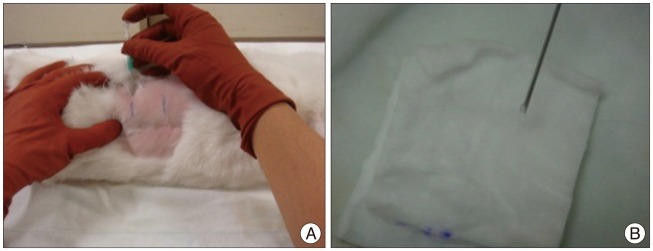
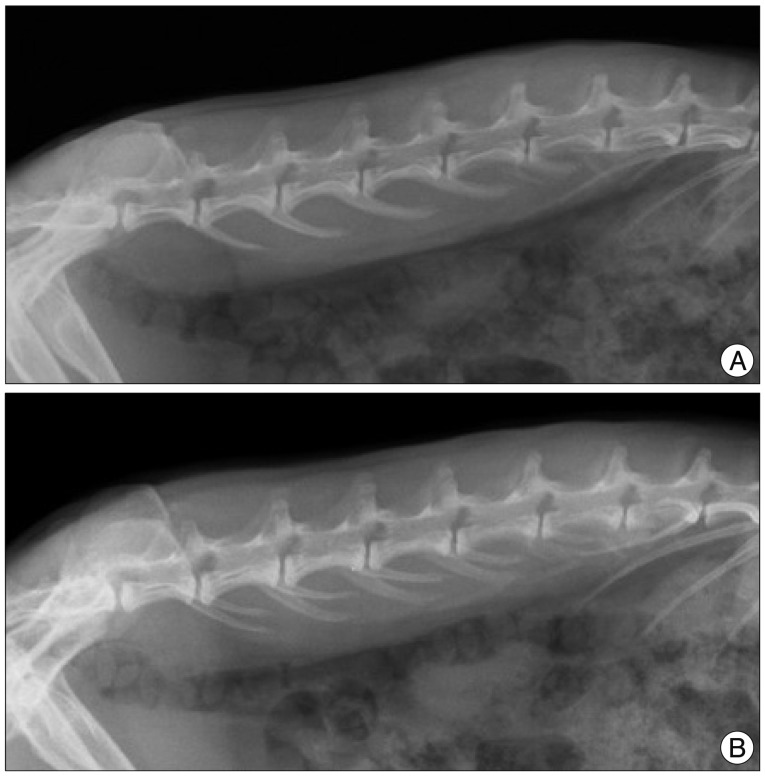
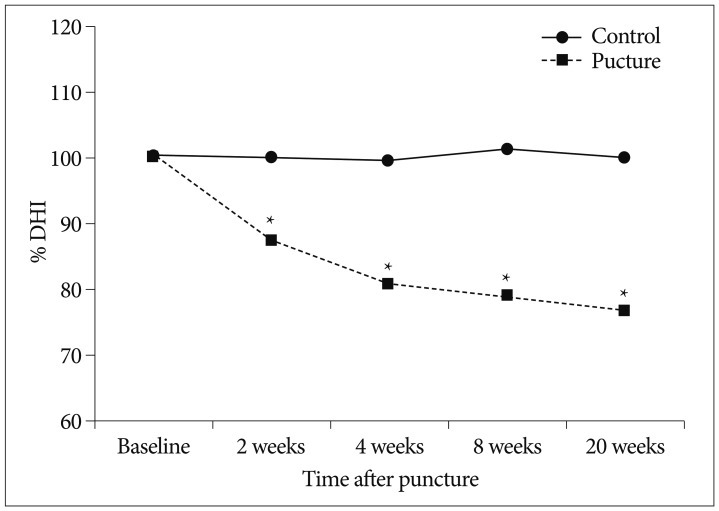
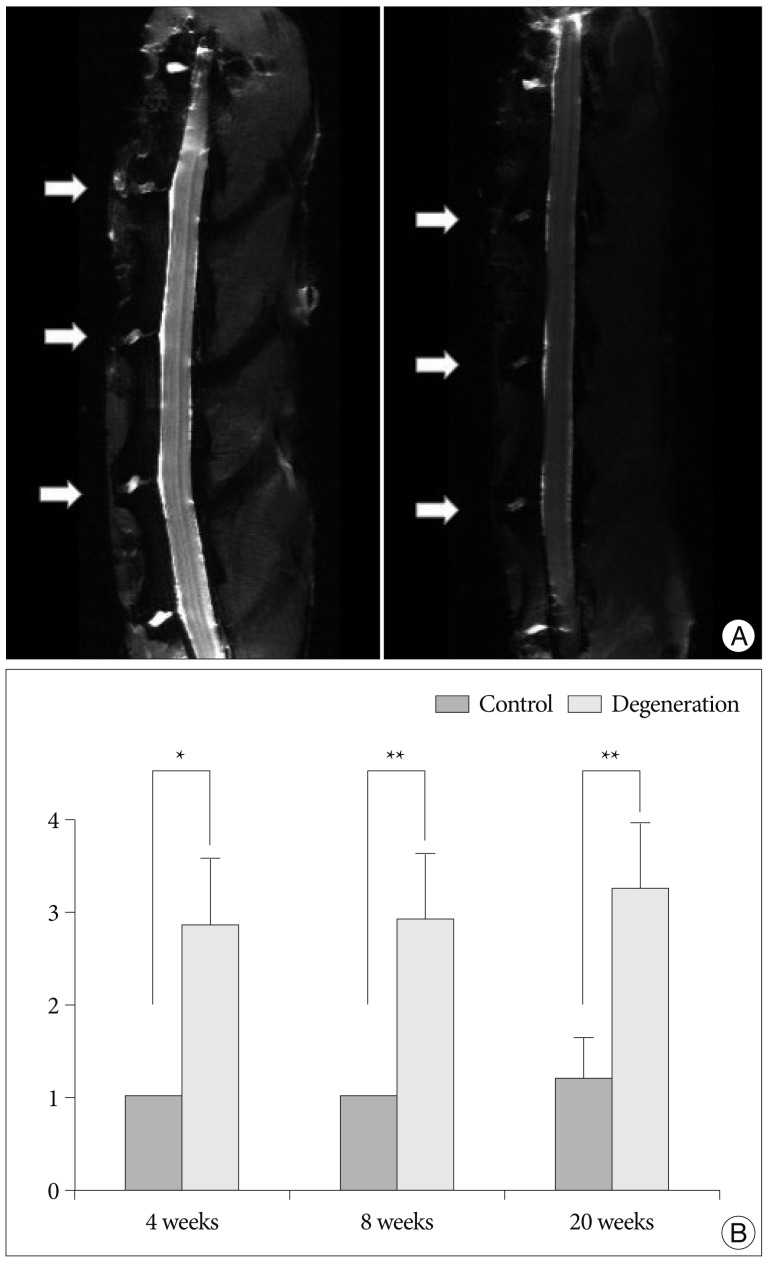
Fifteen New Zealand white rabbits (4 to 5 months old and weighing approximately 3 to 3.5 kg each) underwent annular puncture of the L2-L3, L3-L4, and L4-L5 discs. Rabbits were sacrificed at 4, 8, or 20 weeks after puncture. For a longitudinal study to assess changes in disc height over time, serial X-rays were performed at 0, 2, 4, 8, and 20 weeks for rabbits in the 20-week group. Upon sacrifice, the whole spinal column and discs were extracted and analyzed with magnetic resonance imaging (MRI), real time reverse transcriptase-polymerase chain reaction, and histological staining.
RESULTS
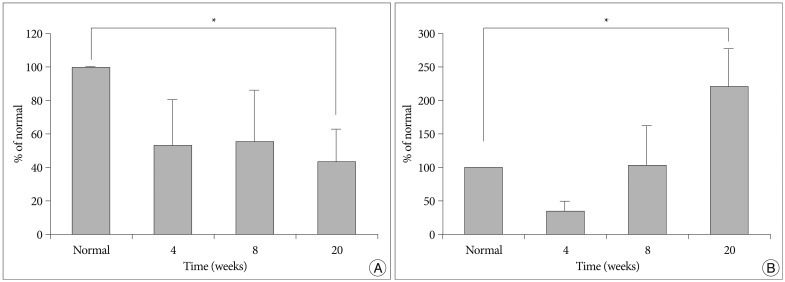
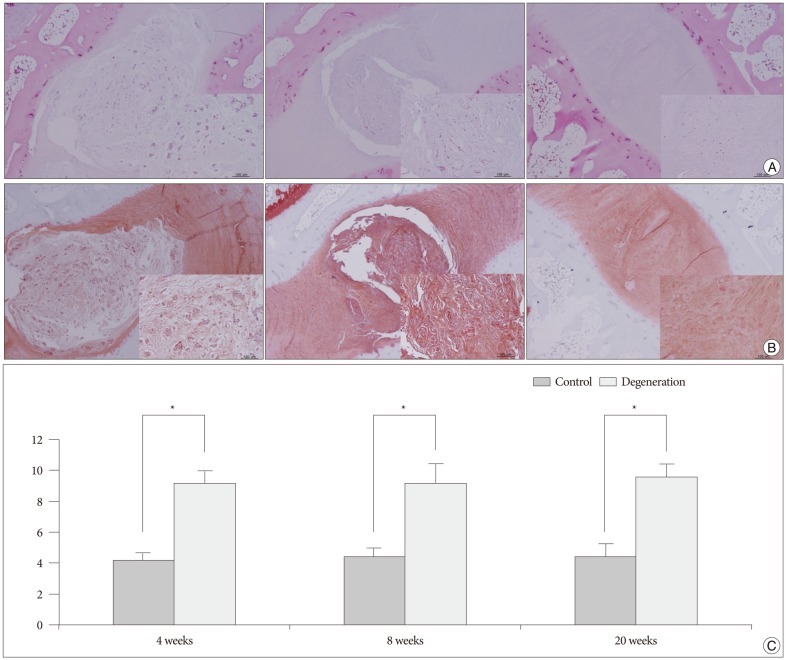
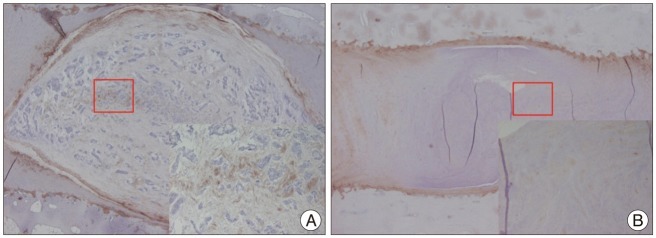
The X-rays showed a slow, progressive decrease in disc height over time. Significant disc space narrowing compared to preoperative disc height was observed during the time period (p<0.001). The MRI grade, aggrecan, and matrix metalloprotease-13 mRNA expression and hematoxylin and eosin/safranin O/anti-collagen II staining were consistently indicative of degeneration, supporting the results of the X-ray data.
CONCLUSION
Percutaneous annular puncture resulted in slow, reproducible disc degeneration that was confirmed by radiology, biochemistry, and histology. This in vivo model can be used to study and evaluate the safety and efficacy of biologic treatments for degenerative disc disease.
MeSH Terms
Figure
Cited by 1 articles
-
Grape seed extract inhibits nucleus pulposus cell apoptosis and attenuates annular puncture induced intervertebral disc degeneration in rabbit model
Ogunlade B., Fidelis O. P., Adelakun S. A., Adedotun O. A.
Anat Cell Biol. 2020;53(3):313-324. doi: 10.5115/acb.20.047.
Reference
-
1. Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006; 31:2909–2917. PMID: 17139221.
Article2. Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 2009; 48:5–10. PMID: 18854342.
Article3. Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008; 17(Suppl 4):492–503. PMID: 19005697.
Article4. Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc : the challenges. Eur Spine J. 2008; 17(Suppl 4):480–491. PMID: 19005701.
Article5. Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976). 2013; 38:E49–E58. PMID: 23124260.
Article6. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004; 204:47–54. PMID: 15307137.
Article7. Lipson SJ, Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine (Phila Pa 1976). 1981; 6:194–210. PMID: 7268542.8. Lü DS, Shono Y, Oda I, Abumi K, Kaneda K. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine (Phila Pa 1976). 1997; 22:1828–1834. discussion 1834-1835. PMID: 9280018.
Article9. Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture : correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005; 30:5–14. PMID: 15626974.
Article10. Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976). 2006; 31:742–754. PMID: 16582847.
Article11. Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics : disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007; 24:5–21. PMID: 16963315.
Article12. Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996; 97:761–768. PMID: 8609233.
Article13. Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer : implications for the clinical management of intervertebral disc disorders. Spine (Phila Pa 1976). 2000; 25:2573–2579. PMID: 11034640.
Article14. Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy : an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976). 1999; 24:2419–2425. PMID: 10626303.15. Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976). 1990; 15:762–767. PMID: 2237626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indirect decompression as a minimally invasive spine surgery
- Development of a Unique Mouse Intervertebral Disc Degeneration Model Using a Simple Novel Tool
- Grape seed extract inhibits nucleus pulposus cell apoptosis and attenuates annular puncture induced intervertebral disc degeneration in rabbit model
- Quantitative Analysis of Disc Degeneration Using Axial T2 Mapping in a Percutaneous Annular Puncture Model in Rabbits
- Preventive and Restorative Responses of Broccoli Sprouts on Biomechanical Structures, Histomorphometric, and Gene Expression Levels of Degenerated Intervertebral Disc in Rabbit Model