J Korean Assoc Oral Maxillofac Surg.
2012 Aug;38(4):204-211. 10.5125/jkaoms.2012.38.4.204.
Biological markers around immediately placed titanium implant in the extraction socket of diabetic and insulin-treated rat maxilla
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. spyo@catholic.ac.kr
- KMID: 2189627
- DOI: http://doi.org/10.5125/jkaoms.2012.38.4.204
Abstract
OBJECTIVES
Dental implants installation in patients with diabetes remains controversial as altered bone healing around implants has been reported. And little is known about the biological factors involved in bone healing around implants. The present study aimed to investigate the biological markers around immediately placed implants in rats with controlled and uncontrolled diabetes.
MATERIALS AND METHODS
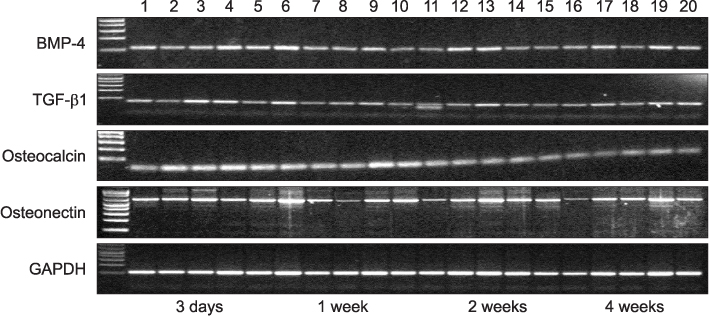
Twenty rats (40 sites) were divided into the control, insulin-treated and diabetic groups. The rats received streptozotocin (60 mg/kg) to induce diabetes; animals in the insulin-treated group also received three units of subcutaneous slow-release insulin. Two threaded titanium alloy implant (1.2x3 mm) were placed in the extraction socket of the both maxillary first molars and allowed for healing. Bone blocks including implant were harvested at 3 days, 1, 2 and 4 weeks. The levels of bone morphogenetic protein (BMP)-4, transforming growth factor (TGF)-beta1, osteocalcin (OC) and osteonectin (ON) were measured in the peri-implant osseous samples by RT-PCR.
RESULTS
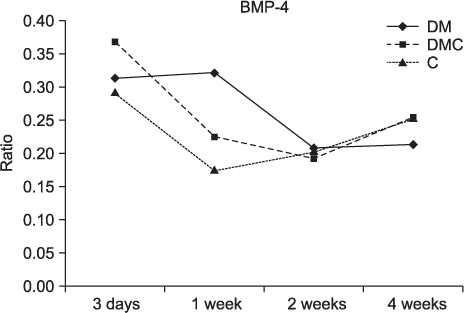
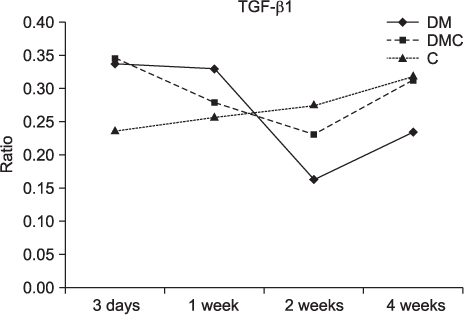
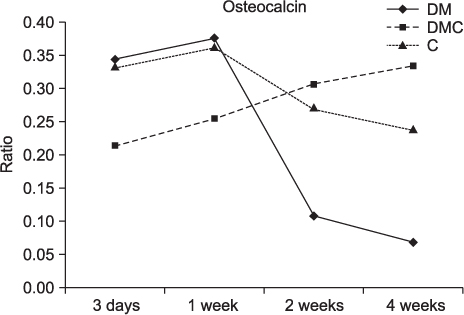
The BMP-4 level increased immediately in all groups by day 3, then decreased abruptly in the control and the insulin-treated groups. However, by week 4, all groups showed mostly the same amount of BMP-4 expression. The level of TGF-beta1 also instantly increased by day 3 in the insulin-treated group. This level elevated again reaching the same values as the control group by week 4, but was not as high as the diabetic group. In addition, the expression of OC and ON in the control and insulin-treated groups was higher than that of the diabetic group at 2 weeks and 4 weeks, indicating active bone formation in these groups.
CONCLUSION
The immediate placement of titanium implants in the maxilla of diabetic rat led to an unwanted bone healing response. Conclusively, the results of this study suggest that immediate implant insertion in patients with poorly controlled diabetes might be contraindicated.
Keyword
MeSH Terms
-
Alloys
Animals
Biological Factors
Biomarkers
Bone Morphogenetic Proteins
Dental Implants
Humans
Insulin
Maxilla
Molar
Osteocalcin
Osteogenesis
Osteonectin
Rats
Streptozocin
Titanium
Transforming Growth Factor beta1
Transforming Growth Factors
Alloys
Biological Factors
Bone Morphogenetic Proteins
Dental Implants
Insulin
Osteocalcin
Osteonectin
Streptozocin
Titanium
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Lazzara RJ. Immediate implant placement into extraction sites: surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989. 9:332–343.2. Wagenberg BD, Ginsburg TR. Immediate implant placement on removal of the natural tooth: retrospective analysis of 1,081 implants. Compend Contin Educ Dent. 2001. 22:399–404.3. Becker W, Goldstein M. Immediate implant placement: treatment planning and surgical steps for successful outcome. Periodontol 2000. 2008. 47:79–89.
Article4. Marder MZ. Medical conditions affecting the success of dental implants. Compend Contin Educ Dent. 2004. 25:739–742.5. Oczakir C, Balmer S, Mericske-stern R. Implant-prosthodontic treatment for special care patients: a case series study. Int J Prosthodont. 2005. 18:383–389.6. McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am. 1995. 9:1–9.
Article7. Kotsovilis S, Karoussis IK, Fourmousis I. A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res. 2006. 17:587–599.
Article8. Mombelli A, Cionca N. Systemic diseases affecting osseointegration therapy. Clin Oral Implants Res. 2006. 17:Suppl 2. 97–103.
Article9. Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants. 2000. 15:811–818.10. van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res. 2002. 13:617–622.
Article11. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000. 20:366–373.12. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000. 5:157–165.
Article13. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998. 13:620–629.
Article14. Fiorellini JP, Nevins ML, Norkin A, Weber HP, Karimbux NY. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res. 1999. 10:362–368.
Article15. Mccracken MS, Aponte-wesson R, Chavali R, Lemons JE. Bone associated with implants in diabetic and insulin-treated rats. Clin Oral Implants Res. 2006. 17:495–500.
Article16. Takeshita F, Iyama S, Ayukawa Y, Kido MA, Murai K, Suetsugu T. The effects of diabetes on the interface between hydroxyapatite implants and bone in rat tibia. J Periodontol. 1997. 68:180–185.
Article17. Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003. 12:242–251.
Article18. Shirakura M, Fujii N, Ohnishi H, Taguchi Y, Ohshima H, Nomura S, et al. Tissue response to titanium implantation in the rat maxilla, with special reference to the effects of surface conditions on bone formation. Clin Oral Implants Res. 2003. 14:687–696.
Article19. Shyng YC, Devlin H, Ou KL. Bone formation around immediately placed oral implants in diabetic rats. Int J Prosthodont. 2006. 19:513–514.20. Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants. 2008. 23:237–246.21. Kwon PT, Rahman SS, Kim DM, Kopman JA, Karimbux NY, Fiorellini JP. Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J Periodontol. 2005. 76:621–626.
Article22. Matin K, Senpuku H, Hanada N, Ozawa H, Ejiri S. Bone regeneration by recombinant human bone morphogenetic protein-2 around immediate implants: a pilot study in rats. Int J Oral Maxillofac Implants. 2003. 18:211–217.23. Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984. 33:825–831.
Article24. Verhaeghe J, Suiker AM, Nyomba BL, Visser WJ, Einhorn TA, Dequeker J, et al. Bone mineral homeostasis in spontaneously diabetic BB rats. II. Impaired bone turnover and decreased osteocalcin synthesis. Endocrinology. 1989. 124:573–582.
Article25. Schenk RK, Buser D. Osseointegration: a reality. Periodontol 2000. 1998. 17:22–35.
Article26. Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001. 83-A Suppl 1:S1–S6.
Article27. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002. 8:147–159.
Article28. Ahrens M, Ankenbauer T, Schröder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993. 12:871–880.
Article29. Ogawa T, Sukotjo C, Nishimura I. Modulated bone matrix-related gene expression is associated with differences in interfacial strength of different implant surface roughness. J Prosthodont. 2002. 11:241–247.
Article30. Carrington JL, Roberts AB, Flanders KC, Roche NS, Reddi AH. Accumulation, localization, and compartmentation of transforming growth factor beta during endochondral bone development. J Cell Biol. 1988. 107:1969–1975.
Article31. Horner A, Kemp P, Summers C, Bord S, Bishop NJ, Kelsall AW, et al. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone. 1998. 23:95–102.
Article32. Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989. 124:2991–2994.
Article33. Rosier RN, O'keefe RJ, Hicks DG. The potential role of transforming growth factor beta in fracture healing. Clin Orthop Relat Res. 1998. 355 Suppl:S294–S300.
Article34. Marcelli C, Yates AJ, Mundy GR. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J Bone Miner Res. 1990. 5:1087–1096.
Article35. Seto H, Aoki K, Kasugai S, Ohya K. Trabecular bone turnover, bone marrow cell development, and gene expression of bone matrix proteins after low calcium feeding in rats. Bone. 1999. 25:687–695.
Article36. Ogawa T, Nishimura I. Genes differentially expressed in titanium implant healing. J Dent Res. 2006. 85:566–570.
Article37. Westerlind KC, Wronski TJ, Evans GL, Turner RT. The effect of long-term ovarian hormone deficiency on transforming growth factor-beta and bone matrix protein mRNA expression in rat femora. Biochem Biophys Res Commun. 1994. 200:283–289.
Article38. Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993. 22:147–159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone response around immediately placed titanium implant in the extraction socket of diabetic and insulin-treated rat maxilla
- Radiographic Bone Density Around Immediately Placed Titanium Implant on the Extraction Socket of Diabetic and Insulin-Treated Rat Maxilla
- COMPARATIVE STUDY ON THE MARGINAL BONE LOSS OF IMMEDIATE NONSUBMERGED AND SUBMERGED ENDOSSEOUS DENTAL IMPLANTS PLACED INTO EXTRACTION SOCKETS OF DOGS
- A STUDY OF BONE APPOSITION AND MARGINAL ALVEOLAR BONE LOSS AROUND IMMEDIATE IMPLANSTS
- Outcome Evaluation of an Immediately Placed Maxillary Anterior Single-Tooth Implant Using Objective Esthetic Criteria: Case Report