J Korean Orthop Assoc.
2007 Jun;42(3):386-394. 10.4055/jkoa.2007.42.3.386.
Osteoinduction using by Human BMP-2-physio- functional Bioactive Ceramics
- Affiliations
-
- 1Department of Orthopaedic Surgery, Brain Korea 21, Yonsei University College of Medicine, Korea. shmoon@yumc.yonsei.ac.kr
- 2Department of Advanced Material Engineering, Yonsei University, Seoul, Korea.
- KMID: 2186565
- DOI: http://doi.org/10.4055/jkoa.2007.42.3.386
Abstract
-
PURPOSE: To assess the properties and the osteogenic potency of the calcium phosphate-recombinant human morphogenetic protein-2 (CaP-rhBMP-2 composite) on glass-ceramics.
MATERIALS AND METHODS
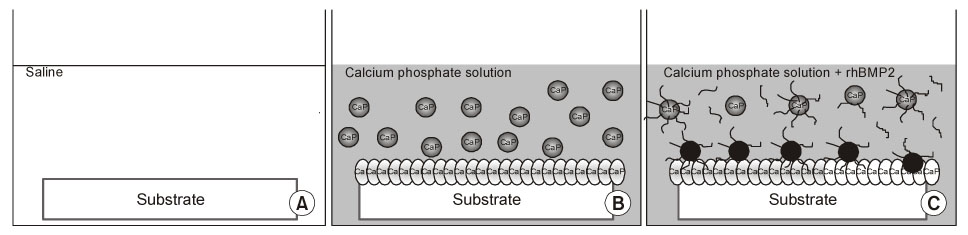
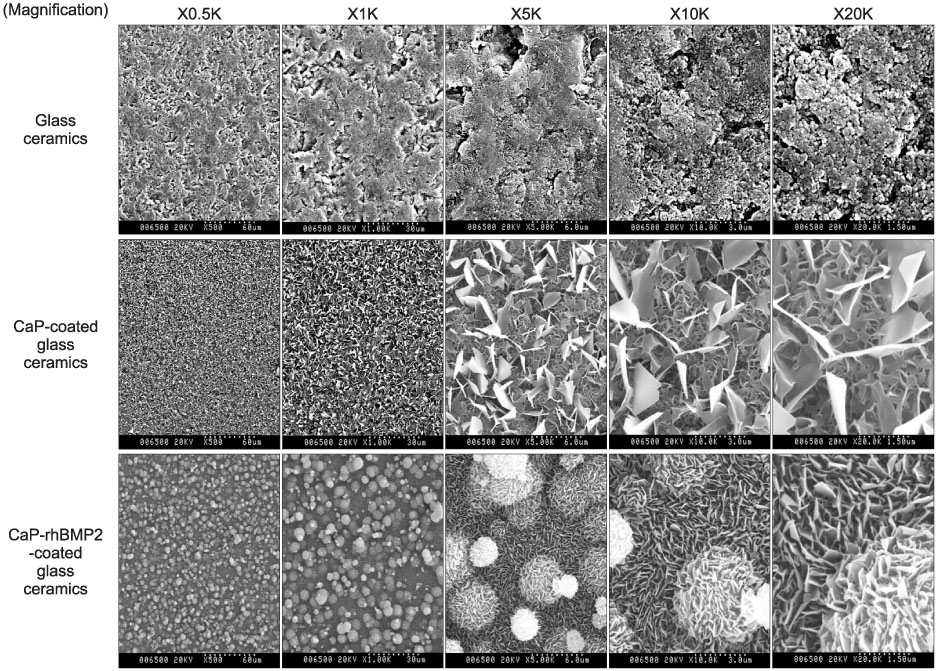
Bioactive glass-ceramics,as a scaffold, and a calcium phosphate (CaP) solution (pH7.4) were prepared. Recombinant human bone morphogenetic protein-2 (rhBMP-2) was purified from CHO-K1 cells by transfecting the cells with BMP-2 cDNA. The glass-ceramics were soaked for 3 days at room temperature in saline, a CaP only solution, and a CaP solution containing rhBMP-2. Scanning electron microscopy (SEM), Fourier transform infrared reflection spectroscopy (FT-IR), thin film X-ray diffraction (TF-XRD) and immunofluorescent staining (IF) of the anti-human BMP-2 to composite-coated scaffold were performed to verify the characterization of the scaffold surface. In addition, RT-PCR of osteogenic marker gene and SEM photography were performed after adhering the mouse preosteoblast MC3T3-E1 cells in order to assess the osteoinductivity.
RESULTS
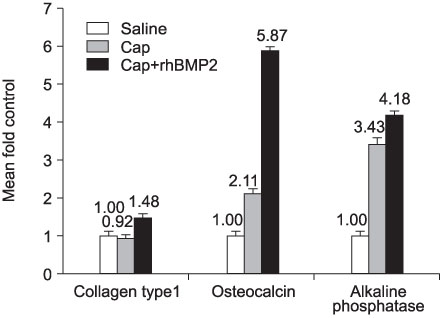
CaP-rhBMP-2 composite was coated on the surface of glass-ceramics, as confirmed by SEM, FT-IR, TF-XRD spectrum, and IF. The CaP-rhBMP-2 composite on the glass-ceramic showed a globular shape covered with fine spikes while the CaP on the glass-ceramic showed a fine spike structure on the flat glass surface. The expression of collagen type I and alkaline phosphatase mRNAs had increased 4 hours after cell seeding. In addition, the level of osteocalcin mRNA expression had increased significantly by 3 days in the CaP-rhBMP-2 composite compared with the control and CaP group. The SEM photographs showed more active filopodia formation in the CaP-rhBMP-2 composite than the other groups. There was extensive newly synthesized extracellular matrix around the osteoblasts and CaP-rhBMP-2 composite nodule.
CONCLUSION
The application of CaP-rhBMP-2 composite-surface coating technique on bioactive glass-ceramic is a powerful tool for osteoinduction.
MeSH Terms
-
Alkaline Phosphatase
Animals
Calcium
Ceramics*
Collagen Type I
DNA, Complementary
Extracellular Matrix
Fourier Analysis
Glass
Humans*
Mice
Microscopy, Electron, Scanning
Osteoblasts
Osteocalcin
Photography
Pseudopodia
RNA, Messenger
Spectrum Analysis
X-Ray Diffraction
Alkaline Phosphatase
Calcium
Ceramics
Collagen Type I
DNA, Complementary
Osteocalcin
RNA, Messenger
Figure
Reference
-
1. Chang MC, Ikoma T, Kikuchi M, Tanaka J. The cross-linkage effect of hydroxyapatite/collagen nanocomposites on a self-organization phenomenon. J Mater Sci Mater Med. 2002. 13:993–997.2. Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991. 2:187–208.
Article3. Grenga TE, Zins JE, Bauer TW. The rate of vascularization of coralline hydroxyapatite. Plast Reconstr Surg. 1989. 84:245–249.
Article4. Hench LL. Bioactive ceramics. Ann N Y Acad Sci. 1988. 523:54–71.
Article5. Hench LL. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res. 1998. 41:511–518.
Article6. Hench LL. Biomaterials: a forecast for the future. Biomaterials. 1998. 19:1419–1423.
Article7. Hench LL, Wilson J. Surface-active biomaterials. Science. 1984. 226:630–636.
Article8. Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed. 2004. 15:543–562.
Article9. Jiang BB, Gao CY, Hu L, Shen JC. Water-dispersed bone morphogenetic protein nanospheres prepared by co-precipitation method. J Zhejiang Univ Sci. 2004. 5:936–940.
Article10. Kamakura S, Nakajo S, Suzuki O, Sasano Y. New scaffold for recombinant human bone morphogenetic protein-2. J Biomed Mater Res A. 2004. 71:299–307.
Article11. Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials. 2005. 26:4023–4034.
Article12. Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001. 22:1705–1711.
Article13. Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991. 12:155–163.
Article14. Krout A, Wen HB, Hippensteel E, Li P. A hybrid coating of biomimetic apatite and osteocalcin. J Biomed Mater Res A. 2005. 73:377–387.
Article15. Langer R, Vacanti JP. Tissue engineering. Science. 1993. 260:920–926.
Article16. Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004. 69:158–165.
Article17. Minamide A, Kawakami M, Hashizume H, Sakata R, Tamaki T. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001. 26:933–939.
Article18. Oonishi H, Kushitani S, Yasukawa E, et al. Particulate bioglass compared with hydroxyapatite as a bone graft substitute. Clin Orthop Relat Res. 1997. 334:316–325.
Article19. Schliephake H, Neukam FW, Klosa D. Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes. A histometric study. Int J Oral Maxillofac Surg. 1991. 20:53–58.20. Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001. 52:443–451.
Article21. Suh DY, Boden SD, Louis-Ugbo J, et al. Delivery of recombinant human bone morphogenetic protein-2 using a compression-resistant matrix in posterolateral spine fusion in the rabbit and in the non-human primate. Spine. 2002. 27:353–360.
Article22. Tancred DC, McCormack BA, Carr AJ. A synthetic bone implant macroscopically identical to cancellous bone. Biomaterials. 1998. 19:2303–2311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regeneration of Artificial Bone Defects by Allograft of Demineralized Bone and Bone Particles in Rabbits
- The effect of purified human BMP with DLB(hBMP-I) on osseointegration of immediate titanium implants: cases report
- Morphological Study for Osteoinduction by Human Fibroblasts Transduced with rhBMP-7 Adenovirus
- BMP‑6 Attenuates Oxygen and Glucose Deprivation-Induced Apoptosis in Human Neural Stem Cells through Inhibiting p38 MAPK Signaling Pathway
- The histologic study of the grafted hBMP-I for immediate implant fixation