Blood Res.
2013 Jun;48(2):99-106. 10.5045/br.2013.48.2.99.
Efficacy of pamidronate in children with low bone mineral density during and after chemotherapy for acute lymphoblastic leukemia and non-Hodgkin lymphoma
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Yeungnam University, Daegu, Korea. johah@med.yu.ac.kr
- KMID: 2172907
- DOI: http://doi.org/10.5045/br.2013.48.2.99
Abstract
- BACKGROUND
Reduced bone mineral density (BMD) is a significant sequelae in children receiving chemotherapy for acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Reduced BMD is associated with an increased risk for fractures. Pamidronate, a second-generation bisphosphonate, has been used to treat osteoporosis in children. This study evaluated the safety and efficacy of pamidronate in children with low BMD during and after chemotherapy for ALL and NHL.
METHODS
Between April 2007 and October 2011, 24 children with ALL and NHL were treated with pamidronate. The indication was a decreased BMD Z-score less than -2.0 or bone pain with a BMD Z-score less than 0. Pamidronate was infused at 1 mg/kg/day for 3 days at 1-4 month intervals (pamidronate group, cases). The BMD Z-scores of the cases were compared with those of 10 untreated patients (control group). Lumbar spine BMDs were measured every 6 cycles using dual energy X-ray absorptiometry and Z-scores were calculated. Bone turnover parameters (25-hydroxyvitamin D, alkaline phosphatase, parathyroid hormone, osteocalcin, and type I collagen c-terminal telopeptide) were analyzed.
RESULTS
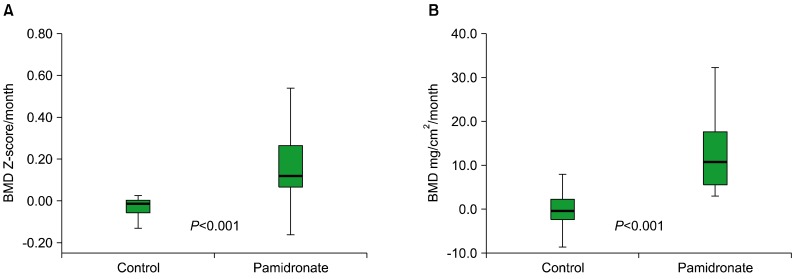
The median cycle of pamidronate treatment was 12. Increases in BMD Z-scores were significantly higher in the pamidronate group than in the control group (P<0.001). BMD (mg/cm2) increased in all pamidronate-treated cases. Twenty patients who complained of bone pain reported pain relief after therapy. The treatment was well tolerated.
CONCLUSION
Pamidronate appears to be safe and effective for the treatment of children with low BMD during and after chemotherapy for ALL and NHL.
Keyword
MeSH Terms
-
Absorptiometry, Photon
Adrenal Cortex Hormones
Alkaline Phosphatase
Bone Density
Child
Collagen Type I
Diphosphonates
Humans
Lymphoma, Non-Hodgkin
Osteocalcin
Osteoporosis
Parathyroid Hormone
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Spine
Adrenal Cortex Hormones
Alkaline Phosphatase
Collagen Type I
Diphosphonates
Osteocalcin
Parathyroid Hormone
Figure
Cited by 2 articles
-
Bone mineral density change during adjuvant chemotherapy in pediatric osteosarcoma
Ju Hyun Ahn, Wan Hyeong Cho, Jun Ah Lee, Dong Ho Kim, Ju-Hee Seo, Jung Sub Lim
Ann Pediatr Endocrinol Metab. 2015;20(3):150-154. doi: 10.6065/apem.2015.20.3.150.Low bone mineral density in children and adolescents with cancer
Hye Young Jin, Jun Ah Lee
Ann Pediatr Endocrinol Metab. 2020;25(3):137-144. doi: 10.6065/apem.2040060.030.
Reference
-
1. Ministry of Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. Seoul, Korea: Ministry of Health, Welfare and Family Affairs;2009.2. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355:1572–1582. PMID: 17035650.
Article3. van der Sluis IM, van den Heuvel-Eibrink MM. Osteoporosis in children with cancer. Pediatr Blood Cancer. 2008; 50(2 Suppl):474–478. PMID: 18064660.
Article4. Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008; 121:e705–e713. PMID: 18310191.
Article5. Benmiloud S, Steffens M, Beauloye V, et al. Long-term effects on bone mineral density of different therapeutic schemes for acute lymphoblastic leukemia or non-Hodgkin lymphoma during childhood. Horm Res Paediatr. 2010; 74:241–250. PMID: 20395671.
Article6. Bianchi ML. How to manage osteoporosis in children. Best Pract Res Clin Rheumatol. 2005; 19:991–1005. PMID: 16301193.
Article7. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339:947–952. PMID: 9753709.
Article8. Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab. 2003; 88:986–992. PMID: 12629073.
Article9. Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004; 35:235–242. PMID: 15207763.
Article10. Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002; 141:644–651. PMID: 12410192.
Article11. Kim SD, Cho BS. Pamidronate therapy for preventing steroid-induced osteoporosis in children with nephropathy. Nephron Clin Pract. 2006; 102:c81–c87. PMID: 16282699.
Article12. Barr RD, Guo CY, Wiernikowski J, Webber C, Wright M, Atkinson S. Osteopenia in children with acute lymphoblastic leukemia: a pilot study of amelioration with pamidronate. Med Pediatr Oncol. 2002; 39:44–46. PMID: 12116079.
Article13. Goldbloom EB, Cummings EA, Yhap M. Osteoporosis at presentation of childhood ALL: management with pamidronate. Pediatr Hematol Oncol. 2005; 22:543–550. PMID: 16166046.14. Lee SH, Desai SS, Shetty G, et al. Bone mineral density of proximal femur and spine in Korean children between 2 and 18 years of age. J Bone Miner Metab. 2007; 25:423–430. PMID: 17968496.
Article15. Högler W, Wehl G, van Staa T, Meister B, Klein-Franke A, Kropshofer G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: comparison of fracture risk with the General Practice Research Database. Pediatr Blood Cancer. 2007; 48:21–27. PMID: 16317756.
Article16. Haddy TB, Mosher RB, Reaman GH. Osteoporosis in survivors of acute lymphoblastic leukemia. Oncologist. 2001; 6:278–285. PMID: 11423675.
Article17. Choi JH, Shin YL, Yoo HW. Short-term efficacy of monthly pamidronate infusion in patients with osteogenesis imperfecta. J Korean Med Sci. 2007; 22:209–212. PMID: 17449925.
Article18. Meyers PA, Healey JH, Chou AJ, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011; 117:1736–1744. PMID: 21472721.
Article19. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007; 119(Suppl 2):S150–S162. PMID: 17332236.
Article20. Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999; 104:1363–1374. PMID: 10562298.
Article21. Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008; 26:3038–3045. PMID: 18565890.
Article22. Kotecha RS, Powers N, Lee SJ, Murray KJ, Carter T, Cole C. Use of bisphosphonates for the treatment of osteonecrosis as a complication of therapy for childhood acute lymphoblastic leukaemia (ALL). Pediatr Blood Cancer. 2010; 54:934–940. PMID: 20127847.
Article23. Greggio NA, Pillon M, Varotto E, et al. Short-term bisphosphonate therapy could ameliorate osteonecrosis: a complication in childhood hematologic malignancies. Case Rep Med. 2010; 2010:206132. PMID: 20589085.
Article24. Swaminathan R. Biochemical markers of bone turnover. Clin Chim Acta. 2001; 313:95–105. PMID: 11694245.
Article25. Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368. PMID: 8854049.
Article26. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams textbook of endocrinology: Expert consult. 12th ed. Philadelphia, PA: Elsevier Health Sciences;2011. p. 1239–1241.27. Van den Wyngaert T, Huizing MT, Vermorken JB. Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol. 2006; 17:1197–1204. PMID: 16873439.
Article28. Mehrotra B, Ruggiero S. Bisphosphonate complications including osteonecrosis of the jaw. Hematology Am Soc Hematol Educ Program. 2006; 356–360. 515PMID: 17124083.
Article29. Bianchi ML, Cimaz R, Bardare M, et al. Efficacy and safety of alendronate for the treatment of osteoporosis in diffuse connective tissue diseases in children: a prospective multicenter study. Arthritis Rheum. 2000; 43:1960–1966. PMID: 11014345.
Article30. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003; 98:1735–1744. PMID: 14534891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of pamidronate in pediatric osteosarcoma patients with low bone mineral density
- Bone morbidity in pediatric acute lymphoblastic leukemia
- Cyclophosphamide-Induced Hemorrhagic Cystitis in Acute Lymphocytic Leukemia and Non-Hodgkin Lymphoma
- A Case of Bone Marrow Necrosis Following Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia
- A case of bone marrow necrosis in acute lymphoblastic leukemia