Ann Pediatr Endocrinol Metab.
2020 Mar;25(1):1-9. 10.6065/apem.2020.25.1.1.
Bone morbidity in pediatric acute lymphoblastic leukemia
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Catholic University of Korea, Seoul, Korea
- KMID: 2501030
- DOI: http://doi.org/10.6065/apem.2020.25.1.1
Abstract
- Acute lymphoblastic leukemia (ALL), currently the most common pediatric leukemia, has a high curability rate of up to 90%. Endocrine disorders are highly prevalent in children with ALL, and skeletal morbidity is a major issue induced by multiple factors associated with ALL. Leukemia itself is a predominant risk factor for decreased bone formation, and major bone destruction occurs secondary to chemotherapeutic agents. Glucocorticoids are cornerstone drugs used throughout the course of ALL treatment that exert significant effects on demineralization and osteoclastogenesis. After completion of treatment, ALL survivors are prone to multiple hormone deficiencies that eventually affect bone mineral accrual. Dual-energy X-ray absorptiometry, the most widely used method of measuring bone mineral density, is used to determine the presence of childhood osteoporosis and vertebral fracture. Supplementation with calcium and vitamin D, administration of pyrophosphate analogues, and promotion of mobility and exercise are effective options to prevent further bone resorption and fracture incidence. This review focuses on addressing bone morbidity after pediatric ALL treatment and provides an overview of bone pathology based on skeletal outcomes to increase awareness among pediatric hemato-oncologists and endocrinologists.
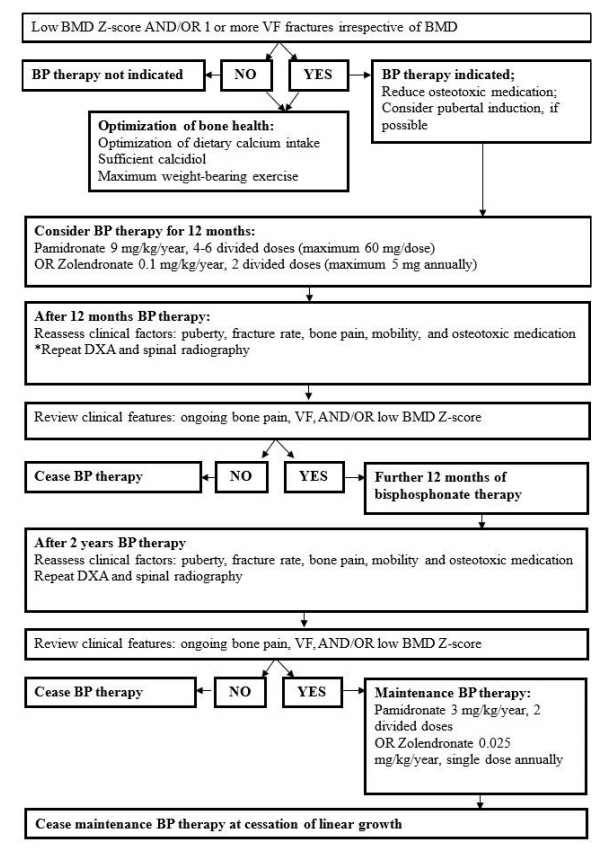
Figure
Reference
-
References
1. Lee JW, Cho B. Prognostic factors and treatment of pediatric acute lymphoblastic leukemia. Korean J Pediatr. 2017; 60:129–37.
Article2. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013; 381:1943–55.
Article3. Demidowicz E, Pogorzala M, Lecka M, Zolnowska H, Marjanska A, Kubicka M, et al. Outcome of pediatric acute lymphoblastic leukemia: sixty years of progress. Anticancer Res. 2019; 39:5203–7.
Article4. Sklar CA, Antal Z, Chemaitilly W, Cohen LE, Follin C, Meacham LR, et al. Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018; 103:2761–84.
Article5. Howard SC, Pui CH. Endocrine complications in pediatric patients with acute lymphoblastic leukemia. Blood Rev. 2002; 16:225–43.
Article6. Mostoufi-Moab S, Ward LM. Skeletal morbidity in children and adolescents during and following cancer therapy. Horm Res Paediatr. 2019; 91:137–51.
Article7. Kizilocak H, Okcu F. Late effects of therapy in childhood acute lymphoblastic leukemia survivors. Turk J Haematol. 2019; 36:1–11.
Article8. Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018; 54:223–33.
Article9. Mostoufi-Moab S, Halton J. Bone morbidity in childhood leukemia: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. 2014; 12:300–12.
Article10. Warner JT, Evans WD, Webb DK, Bell W, Gregory JW. Relative osteopenia after treatment for acute lymphoblastic leukemia. Pediatr Res. 1999; 45:544–51.
Article11. Davies JH, Evans BA, Jenney ME, Gregory JW. Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin Endocrinol (Oxf). 2005; 63:1–9.
Article12. Shimo T, Sasaki A. Mechanism of cancer-induced bone destruction: an association of connective tissue growth factor (CTGF/CCN2) in the bone metastasis. Japanese Dental Science Review. 2011; 47:13–22.
Article13. Mattia C, Coluzzi F, Celidonio L, Vellucci R. Bone pain mechanism in osteoporosis: a narrative review. Clin Cases Miner Bone Metab. 2016; 13:97–100.
Article14. Angsubhakorn N, Suvannasankha A. Acute lymphoblastic leukaemia with osteolytic bone lesions: diagnostic dilemma. BMJ Case Rep. 2018; Aug. 11. ;2018. pii: bcr-2018-225008. doi: 10.1136/bcr-2018-225008.
Article15. Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, dxa cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019; 22:453–71.
Article16. Cummings EA, Ma J, Fernandez CV, Halton J, Alos N, Miettunen PM, et al. Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab. 2015; 100:3408–17.
Article17. Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009; 24:1326–34.
Article18. Carriere B, Cummins-Mcmanus B. Vertebral fractures as initial signs for acute lymphoblastic leukemia. Pediatr Emerg Care. 2001; 17:258–61.
Article19. Stagi S, Cavalli L, Iurato C, Seminara S, Brandi ML, de Martino M. Bone health in children and adolescents: the available imaging techniques. Clin Cases Miner Bone Metab. 2013; 10:166–71.20. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993; 8:1137–48.
Article21. Koerber F, Schulze Uphoff U, Koerber S, Schonau E, Maintz D, Semler O. Introduction of a new standardized assessment score of spine morphology in osteogenesis imperfecta. Rofo. 2012; 184:719–25.
Article22. Kim YM, Demissie S, Eisenberg R, Samelson EJ, Kiel DP, Bouxsein ML. Intra-and inter-reader reliability of semiautomated quantitative morphometry measurements and vertebral fracture assessment using lateral scout views from computed tomography. Osteoporos Int. 2011; 22:2677–88.
Article23. Alqahtani FF, Offiah AC. Diagnosis of osteoporotic vertebral fractures in children. Pediatr Radiol. 2019; 49:283–96.
Article24. Levine MA. Assessing bone health in children and adolescents. Indian J Endocrinol Metab. 2012; 16(Suppl 2):S205–12.
Article25. Stagi S, Cavalli L, Cavalli T, de Martino M, Brandi ML. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: a review. Ital J Pediatr. 2016; 42:88.
Article26. Zemel BS. Quantitative computed tomography and computed tomography in children. Curr Osteoporos Rep. 2011; 9:284–90.
Article27. Fewtrell MS, British P, Adolescent Bone G. Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child. 2003; 88:795–8.
Article28. Shaw N, Crabtree N. Bone density in children: what are we measuring? Arch Dis Child. 2019; 104:1108–11.
Article29. Adams JE. Bone densitometry in children. Semin Musculoskelet Radiol. 2016; 20:254–68.
Article30. Adesina OO, Gurney JG, Kang G, Villavicencio M, Hodges JR, Chemaitilly W, et al. Height-corrected low bone density associates with severe outcomes in sickle cell disease: SCCRIP cohort study results. Blood Adv. 2019; 3:1476–88.
Article31. Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, et al. Bone mineral density according to age, bone age, and pubertal stages in korean children and adolescents. J Clin Densitom. 2010; 13:68–76.
Article32. Orgel E, Mueske NM, Wren TA, Gilsanz V, Butturini AM, Freyer DR, et al. Early injury to cortical and cancellous bone from induction chemotherapy for adolescents and young adults treated for acute lymphoblastic leukemia. Bone. 2016; 85:131–7.
Article33. Pufall MA. Glucocorticoids and cancer. Adv Exp Med Biol. 2015; 872:315–33.
Article34. Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010; 11:1096–106.
Article35. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). 1932. Obes Res. 1994; 2:486–508.
Article36. Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J Endocrinol. 2000; 164:339–48.
Article37. O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004; 145:1835–41.38. Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006; 147:5592–9.
Article39. Tack LJ, Tatsi C, Stratakis CA, Lodish MB. Effects of glucocorticoids on bone: what we can learn from pediatric endogenous Cushing's syndrome. Horm Metab Res. 2016; 48:764–70.
Article40. Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. 2014; 44:47–54.
Article41. Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015; 1:e000014.
Article42. Ward LM. Osteoporosis due to glucocorticoid use in children with chronic illness. Horm Res. 2005; 64:209–21.
Article43. Wilson CL, Ness KK. Bone mineral density deficits and fractures in survivors of childhood cancer. Curr Osteoporos Rep. 2013; 11:329–37.
Article44. Lentle B, Ma J, Jaremko JL, Siminoski K, Matzinger MA, Shenouda N, et al. The radiology of vertebral fractures in childhood osteoporosis related to glucocorticoid administration. J Clin Densitom. 2016; 19:81–8.
Article45. te Winkel ML, Pieters R, Hop WC, Roos JC, Bokkerink JP, Leeuw JA, et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the DCOG-ALL9 protocol. Bone. 2014; 59:223–8.
Article46. Ma J, Siminoski K, Alos N, Halton J, Ho J, Cummings EA, et al. Impact of vertebral fractures and glucocorticoid exposure on height deficits in children during treatment of leukemia. J Clin Endocrinol Metab. 2019; 104:213–22.
Article47. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007; 65:168–73.48. Crofton PM, Ahmed SF, Wade JC, Elmlinger MW, Ranke MB, Kelnar CJ, et al. Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Pediatr Res. 2000; 48:490–6.
Article49. Atkinson SA, Fraher L, Gundberg CM, Andrew M, Pai M, Barr RD. Mineral homeostasis and bone mass in children treated for acute lymphoblastic leukemia. J Pediatr. 1989; 114:793–800.
Article50. Egler RA, Ahuja SP, Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother. 2016; 7:62–71.
Article51. Liu C, Janke LJ, Kawedia JD, Ramsey LB, Cai X, Mattano LA Jr, et al. Asparaginase potentiates glucocorticoidinduced osteonecrosis in a mouse model. PLoS One. 2016; 11:e0151433.
Article52. Nissinen TA, Degerman J, Rasanen M, Poikonen AR, Koskinen S, Mervaala E, et al. Systemic blockade of ACVR2B ligands prevents chemotherapy-induced muscle wasting by restoring muscle protein synthesis without affecting oxidative capacity or atrogenes. Sci Rep. 2016; 6:32695.
Article53. Davies JH, Evans BA, Jenney ME, Gregory JW. Effects of chemotherapeutic agents on the function of primary human osteoblast-like cells derived from children. J Clin Endocrinol Metab. 2003; 88:6088–97.
Article54. Kang MJ, Lim JS. Bone mineral density deficits in childhood cancer survivors: pathophysiology, prevalence, screening, and management. Korean J Pediatr. 2013; 56:60–7.
Article55. Gilsanz V, Carlson ME, Roe TF, Ortega JA. Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. The Journal of Pediatrics. 1990; 117:238–44.
Article56. Nysom K, Holm K, Michaelsen KF, Hertz H, Muller J, Molgaard C. Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol. 1998; 16:3752–60.
Article57. Kaste SC, Jones-Wallace D, Rose SR, Boyett JM, Lustig RH, Rivera GK, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia. 2001; 15:728–34.
Article58. Cox CL, Zhu L, Kaste SC, Srivastava K, Barnes L, Nathan PC, et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018; 65:e26929.
Article59. Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, et al. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res. 2018; 33:1435–43.
Article60. Vitanza NA, Hogan LE, Zhang G, Parker RI. The progression of bone mineral density abnormalities after chemotherapy for childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2015; 37:356–61.
Article61. Mostoufi-Moab S, Brodsky J, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel B, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012; 97:3584–92.
Article62. von Scheven E, Corbin KJ, Stagi S, Cimaz R. Glucocorticoidassociated osteoporosis in chronic inflammatory diseases: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. 2014; 12:289–99.
Article63. Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000; 85:3951–63.64. Grover M, Bachrach LK. Osteoporosis in children with chronic illnesses: diagnosis, monitoring, and treatment. Curr Osteoporos Rep. 2017; 15:271–82.
Article65. Kaste SC, Qi A, Smith K, Surprise H, Lovorn E, Boyett J, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2014; 61:885–93.
Article66. Jayasena A, Atapattu N, Lekamwasam S. Treatment of glucocorticoid-induced low bone mineral density in children: a systematic review. Int J Rheum Dis. 2015; 18:287–93.
Article67. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008; 122:1142–52.
Article68. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006; 117:578–85.
Article69. Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008; 4:827–36.
Article70. Saraff V, Hogler W. Endocrinology and adolescence: osteoporosis in children: diagnosis and management. Eur J Endocrinol. 2015; 173:R185–97.
Article71. Cheung MS. Drugs used in paediatric bone and calcium disorders. Endocr Dev. 2015; 28:277–90.
Article72. Simioni C, Zauli G, Martelli AM, Vitale M, Ultimo S, Milani D, et al. Physical training interventions for children and teenagers affected by acute lymphoblastic leukemia and related treatment impairments. Oncotarget. 2018; 9:17199–209.
Article73. Lucia A, Ramirez M, San Juan AF, Fleck SJ, Garcia-Castro J, Madero L. Intrahospital supervised exercise training: a complementary tool in the therapeutic armamentarium against childhood leukemia. Leukemia. 2005; 19:1334–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of bone marrow necrosis in acute lymphoblastic leukemia
- A Case of Bone Marrow Necrosis Following Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia
- A case of acute lymphoblastic leukemia complicating neuroblastoma in remission
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- A Case of Pediatric Acute Lymphoblastic Leukemia with Trisomy 5 as a Sole Chromosomal Anomaly: A Prognostic Significance