Blood Res.
2015 Jun;50(2):103-108. 10.5045/br.2015.50.2.103.
Human coagulation factor VIII domain-specific recombinant polypeptide expression
- Affiliations
-
- 1Department of Biological Science, College of Natural Sciences, Ajou University, Suwon, Korea. hsunkim@ajou.ac.kr
- 2National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA.
- KMID: 2172774
- DOI: http://doi.org/10.5045/br.2015.50.2.103
Abstract
- BACKGROUND
Hemophilia A is caused by heterogeneous mutations in F8. Coagulation factor VIII (FVIII), the product of F8, is composed of multiple domains designated A1-A2-B-A3-C1-C2. FVIII is known to interact with diverse proteins, and this characteristic may be important for hemostasis. However, little is known about domain-specific functions or their specific binding partners.
METHODS
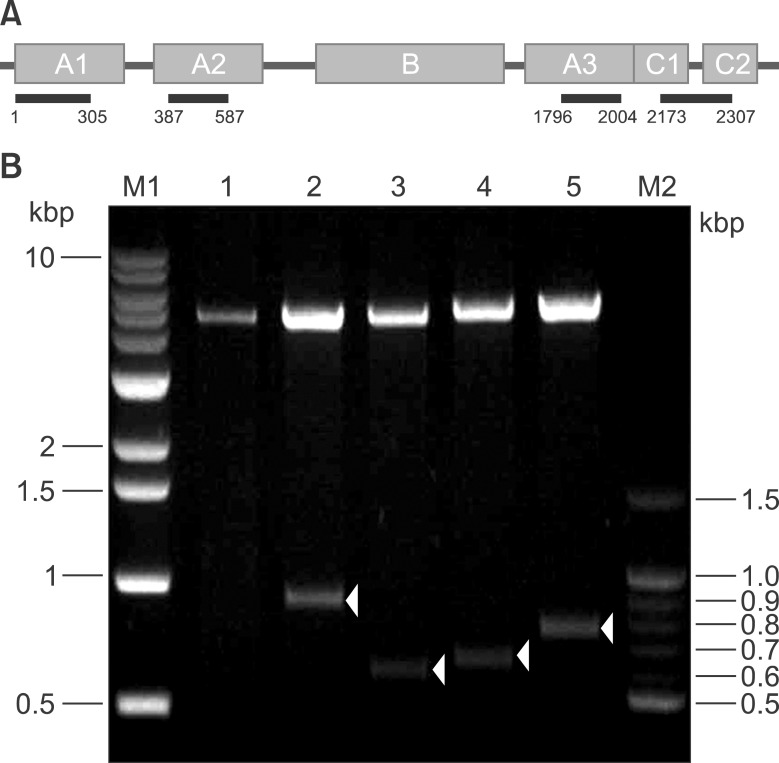
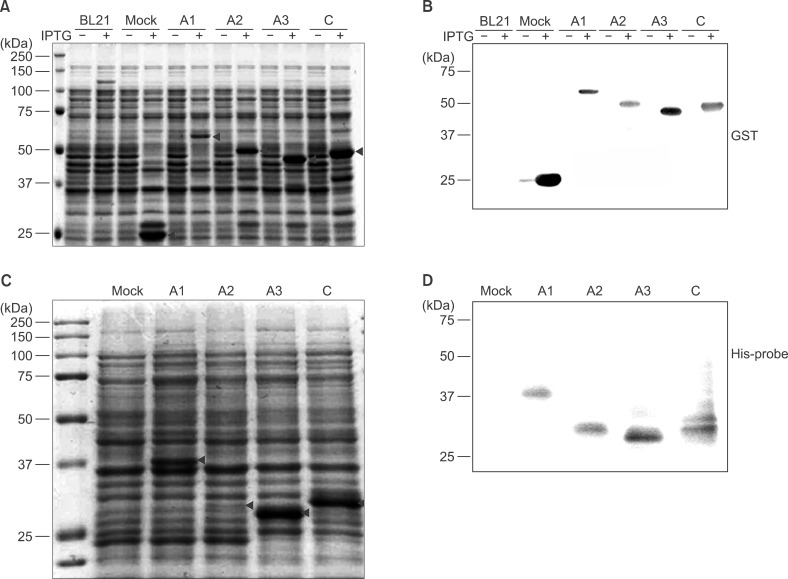
To determine F8 domain-specific functions during blood coagulation, the FVIII domains A1, A2, A3, and C were cloned from Hep3B hepatocytes. Domain-specific recombinant polypeptides were glutathione S-transferase (GST)- or polyhistidine (His)-tagged, over-expressed in bacteria, and purified by specific affinity chromatography.
RESULTS
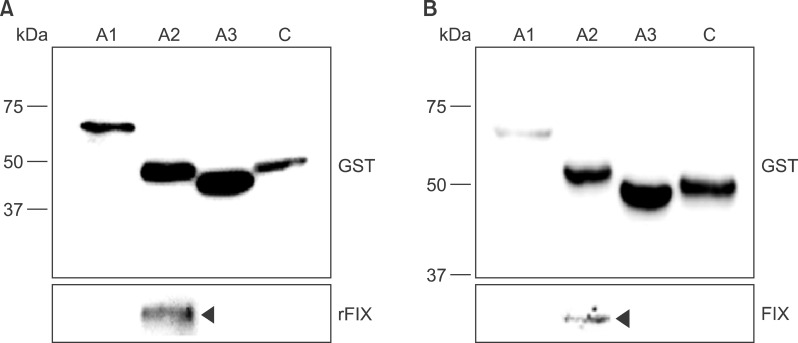
Recombinant polypeptides of predicted sizes were obtained. The GST-tagged A2 polypeptide interacted with coagulation factor IX, which is known to bind the A2 domain of activated FVIII.
CONCLUSION
Recombinant, domain-specific polypeptides are useful tools to study the domain-specific functions of FVIII during the coagulation process, and they may be used for production of domain-specific antibodies.
Keyword
MeSH Terms
Figure
Reference
-
1. Björkman S, Carlsson M, Berntorp E, Stenberg P. Pharmacokinetics of factor VIII in humans. Obtaining clinically relevant data from comparative studies. Clin Pharmacokinet. 1992; 22:385–395. PMID: 1505144.2. Bowen DJ. Haemophilia A and haemophilia B: molecular insights. Mol Pathol. 2002; 55:127–144. PMID: 11950963.
Article3. Pipe SW, Kaufman RJ. Factor VIII C2 domain missense mutations exhibit defective trafficking of biologically functional proteins. J Biol Chem. 1996; 271:25671–25676. PMID: 8810344.
Article4. Fang H, Wang L, Wang H. The protein structure and effect of factor VIII. Thromb Res. 2007; 119:1–13. PMID: 16487577.
Article5. Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998; 92:3983–3996. PMID: 9834200.
Article6. De Brasi CD, Slavutsky IR, Larripa IB. Molecular genetics of hemophilia A. Medicina (B Aires). 1996; 56:509–517. PMID: 9239887.7. Miners AH, Sabin CA, Tolley KH, Lee CA. The changing patterns of factor VIII (FVIII) and factor IX (FIX) clotting factor usage in a comprehensive care centre between 1980 and 1994. Haemophilia. 1998; 4:4–9. PMID: 9873858.
Article8. van't Veer C, Hackeng TM, Delahaye C, Sixma JJ, Bouma BN. Activated factor X and thrombin formation triggered by tissue factor on endothelial cell matrix in a flow model: effect of the tissue factor pathway inhibitor. Blood. 1994; 84:1132–1142. PMID: 8049429.9. Pipe SW. Functional roles of the factor VIII B domain. Haemophilia. 2009; 15:1187–1196. PMID: 19473417.
Article10. Pipe SW, Morris JA, Shah J, Kaufman RJ. Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J Biol Chem. 1998; 273:8537–8544. PMID: 9525969.
Article11. Hwang SH, Kim MJ, Lim JA, Kim HC, Kim HS. Profiling of factor VIII mutations in Korean haemophilia A. Haemophilia. 2009; 15:1311–1317. PMID: 19719548.12. Seo JY, Jang MA, Kim HJ, Lee KO, Kim SH, Kim HJ. Sequence variation data of F8 and F9 genes in functionally validated control individuals: implications on the molecular diagnosis of hemophilia. Blood Res. 2013; 48:206–210. PMID: 24086941.13. Hwang SH, Lim JA, Kim MJ, et al. Profiling of differentially expressed genes in haemophilia A with inhibitor. Haemophilia. 2012; 18:e247–e253. PMID: 22176207.
Article14. Lollar P, Parker CG. Subunit structure of thrombin-activated porcine factor VIII. Biochemistry. 1989; 28:666–674. PMID: 2496750.
Article15. Davidson CJ, Hirt RP, Lal K, et al. Molecular evolution of the vertebrate blood coagulation network. Thromb Haemost. 2003; 89:420–428. PMID: 12624623.
Article16. Hoffman M, Monroe DM 3rd, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998; 9(Suppl 1):S61–S65. PMID: 9819030.17. Kappelmayer J, Bernabei A, Edmunds LH Jr, Edgington TS, Colman RW. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ Res. 1993; 72:1075–1081. PMID: 8097439.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hemophilia A Diagnosed in a Premature Infant
- Synthesis of recombinant blood coagulation factor VIII (FVIII) heavy and light chains and reconstitution of active form of FVIII
- Recurrent Cerebral Venous Thrombosis Associated with Elevated Factor VIII
- Spontaneous Retroperitoneal Hemorrhage Caused by Idiopathic Acquired Hemophilia A Misdiagnosed as a Delayed Traumatic Hematoma: A Case Report
- Post-operative Bleeding due to Acquired Hemophilia Successfully Treated with Recombinant Factor VIIa: Case Report