Chonnam Med J.
2012 Aug;48(2):116-122. 10.4068/cmj.2012.48.2.116.
The Effect of Melatonin on Retinal Ganglion Cell Survival in Ischemic Retina
- Affiliations
-
- 1Department of Ophthalmology, Medical School & Research Institute of Medical Science, Chonnam National University, Gwangju, Korea. exo70@naver.com
- KMID: 2172213
- DOI: http://doi.org/10.4068/cmj.2012.48.2.116
Abstract
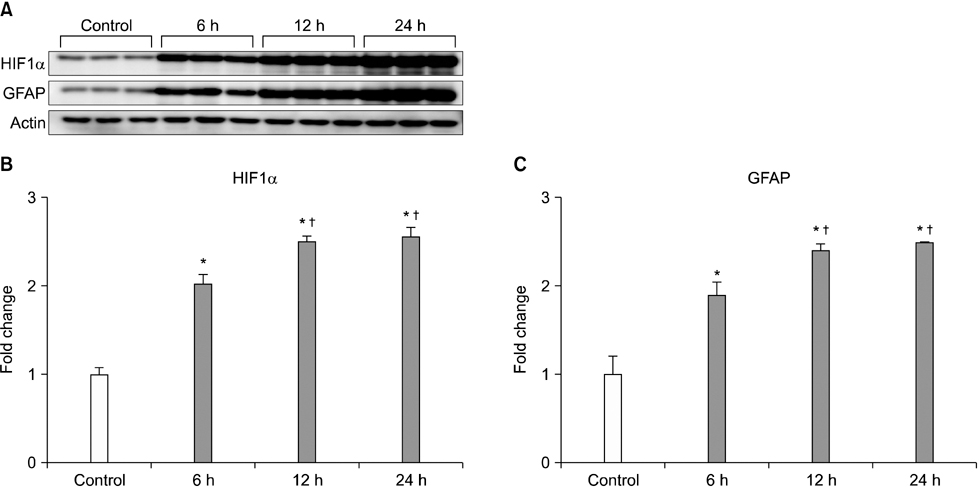
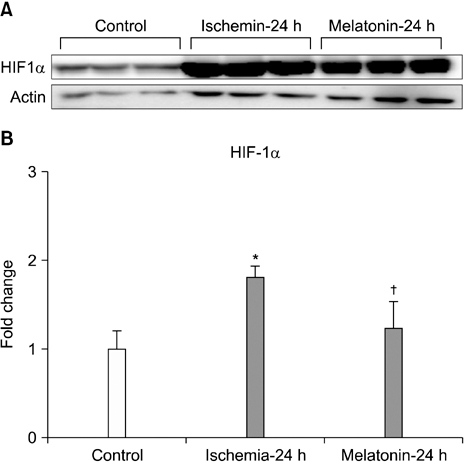
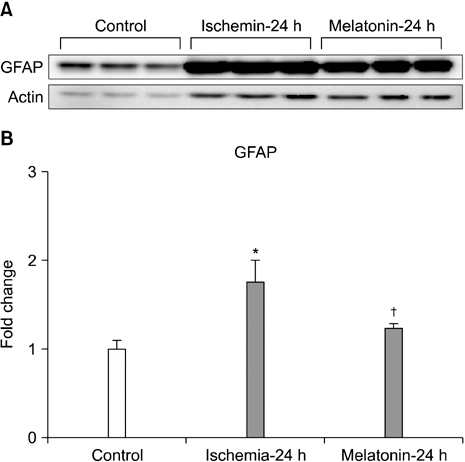
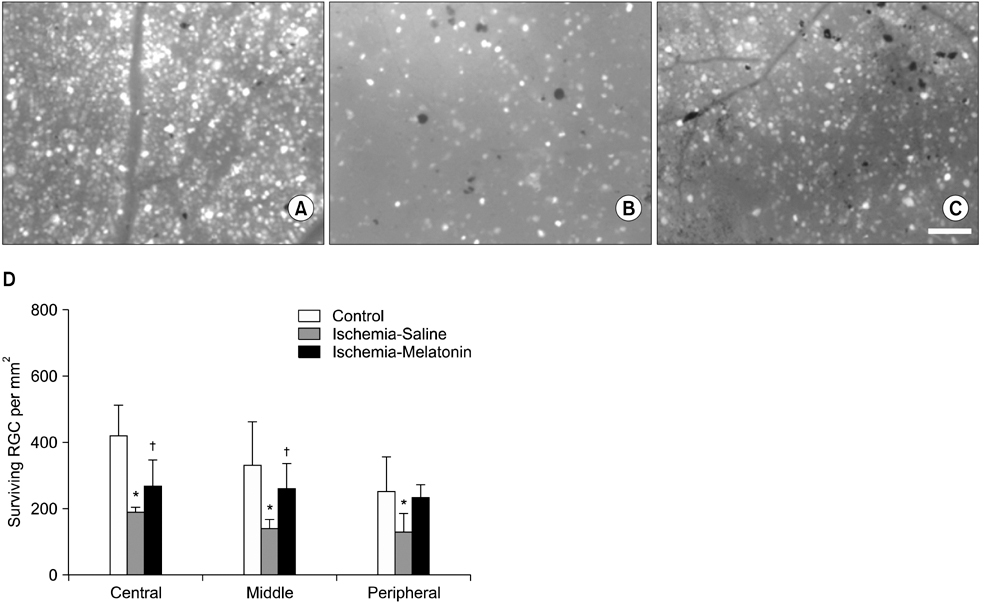
- Our objective was to determine whether melatonin increases retinal ganglion cell (RGC) survival in ischemic mouse retina. Transient retinal ischemia was induced by an acute elevation of intraocular pressure in C57BL/6 mice. To evaluate the effect of melatonin on retinal ischemia, an equal amount of either melatonin or vehicle was intraperitoneally injected into the mice 1 hour before ischemia, at the time of ischemia, and 1 hour after ischemia. Hypoxia inducible factor 1alpha (HIF-1alpha) and glial fibrillary acidic protein (GFAP) expression were assessed 6, 12, and 24 hours after ischemia-reperfusion by Western blot. RGC survival was measured 2 weeks after ischemia-reperfusion. The expression of HIF-1alpha and GFAP peaked 24 hours after ischemia-reperfusion in ischemic retina. The treatment of ischemic retina with melatonin resulted in the inhibition of increased expression of HIF-1alpha and GFAP. RGC survival was greater in retinas treated with melatonin than in retinas treated with vehicle 2 weeks after ischemia-reperfusion. On the basis of our results, we suggest that melatonin treatment increased RGC survival in ischemic mouse retina. The neuroprotective effect of melatonin is mediated by the inhibition of HIF-1alpha stabilization and reduced activity of glial cells in ischemic mouse retina.
Keyword
MeSH Terms
Figure
Reference
-
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996. 80:389–393.
Article2. Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979. 86:1803–1830.
Article3. Anderson DR. What happens to the optic disc and retina in glaucoma? Ophthalmology. 1983. 90:766–770.
Article4. Kelly MEM, Barnes S. Physiology and pathophysiology of nitric oxide in the retina. Neuroscientist. 1997. 3:357–360.
Article5. Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999. 43:Suppl 1. S151–S161.6. Sucher NJ, Lei SZ, Lipton SA. Calcium channel antagonists attenuate NMDA receptor-mediated neurotoxicity of retinal ganglion cells in culture. Brain Res. 1991. 551:297–302.
Article7. Osborne NN, Chidlow G, Nash MS, Wood JP. The potential of neuroprotection in glaucoma treatment. Curr Opin Ophthalmol. 1999. 10:82–92.
Article8. Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999. 43:Suppl 1. S102–S128.
Article9. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004. 363:1711–1720.
Article10. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999. 18:39–57.
Article11. Chung HS, Harris A, Evans DW, Kagemann L, Garzozi HJ, Martin B. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999. 43:Suppl 1. S43–S50.
Article12. Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004. 122:1348–1356.
Article13. Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001. 42:1273–1276.14. Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005. 46:3177–3187.
Article15. Moreno MC, Campanelli J, Sande P, Sánez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004. 37:803–812.
Article16. Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006. 25:490–513.
Article17. Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007. 55:942–953.
Article18. Kim IB, Kim KY, Joo CK, Lee MY, Oh SJ, Chung JW, et al. Reaction of Müller cells after increased intraocular pressure in the rat retina. Exp Brain Res. 1998. 121:419–424.
Article19. Osborne NN, Block F, Sontag KH. Reduction of ocular blood flow results in glial fibrillary acidic protein (GFAP) expression in rat retinal Müller cells. Vis Neurosci. 1991. 7:637–639.
Article20. Fletcher EL, Downie LE, Ly A, Ward MM, Batcha AH, Puthussery T, et al. A review of the role of glial cells in understanding retinal disease. Clin Exp Optom. 2008. 91:67–77.
Article21. Francke M, Faude F, Pannicke T, Uckermann O, Weick M, Wolburg H, et al. Glial cell-mediated spread of retinal degeneration during detachment: a hypothesis based upon studies in rabbits. Vision Res. 2005. 45:2256–2267.
Article22. Kanamori A, Nakamura M, Nakanishi Y, Yamada Y, Negi A. Long-term glial reactivity in rat retinas ipsilateral and contralateral to experimental glaucoma. Exp Eye Res. 2005. 81:48–56.
Article23. Kawasaki A, Otori Y, Barnstable CJ. Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci. 2000. 41:3444–3450.24. Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci USA. 1998. 95:4663–4666.
Article25. Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996. 19:307–312.
Article26. Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006. 25:397–424.
Article27. Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T. Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol. 2000. 44:235–244.
Article28. Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009. 54:143–160.
Article29. Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005. 27:119–130.
Article30. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med. 1996. 21:307–315.31. Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995. 19:123–126.
Article32. Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ. Effects of melatonin on the nitric oxide treated retina. Br J Ophthalmol. 2004. 88:1078–1081.
Article33. Adachi M, Takahashi K, Nishikawa M, Miki H, Uyama M. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch Clin Exp Ophthalmol. 1996. 234:445–451.
Article34. Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol. 2001. 428:185–192.
Article35. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995. 270:1230–1237.
Article36. Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997. 272:22642–22647.
Article37. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999. 15:551–578.38. Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999. 19:6818–6824.
Article39. Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008. 283:10892–10903.
Article40. Akiyama H, Nakazawa T, Shimura M, Tomita H, Tamai M. Presence of mitogen-activated protein kinase in retinal Müller cells and its neuroprotective effect ischemia-reperfusion injury. Neuroreport. 2002. 13:2103–2107.
Article41. Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, et al. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci. 2004. 26:493–502.
Article42. Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003. 44:3025–3033.
Article43. Bignami A, Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979. 28:63–69.
Article44. Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol. 2001. 132:551–556.
Article45. Napper GA, Pianta MJ, Kalloniatis M. Reduced glutamate uptake by retinal glial cells under ischemic/hypoxic conditions. Vis Neurosci. 1999. 16:149–158.
Article46. Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000. 20:8693–8700.
Article47. Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP. Role of melatonin in the eye and ocular dysfunctions. Vis Neurosci. 2006. 23:853–862.
Article48. Agorastos A, Huber CG. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res. 2011. 50:1–7.
Article49. Alonso-Alconada D, Alvarez A, Lacalle J, Hilario E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol Histopathol. 2012. 27:771–783.50. Balduini W, Carloni S, Perrone S, Bertrando S, Tataranno ML, Negro S, et al. The use of melatonin in hypoxic-ischemic brain damage: an experimental study. J Matern Fetal Neonatal Med. 2012. 25:Suppl 1. 119–124.
Article51. Osborne NN, Nash MS, Wood JP. Melatonin counteracts ischemia-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1998. 39:2374–2383.52. Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002. 86:1053–1057.
Article53. Sáenz DA, Goldin AP, Minces L, Chianelli M, Sarmiento MI, Rosenstein RE. Effect of melatonin on the retinal glutamate/glutamine cycle in the golden hamster retina. FASEB J. 2004. 18:1912–1913.
Article54. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrine J. 1993. 1:57–60.55. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004. 36:1–9.
Article56. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005. 38:1–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuroprotective Effect of Intravitreal Melatonin Injection in Pressure-induced Retinal Ischemia
- The Changes of the Retinal Ganglional Cells in the Pressure-induced Ischemic Rabbit Retina
- The Effect of Intravitreal Melatonin on Rabbit Retina
- Expression of the Na(+)-K(+)-2Cl(-)-Cotransporter 2 in the Normal and Pressure-Induced Ischemic Rat Retina
- Characteristics of Light-evoked Retinal Ganglion Cell Activity with Postnatal Maturation in SD Rat