Korean J Ophthalmol.
2012 Jun;26(3):203-211. 10.3341/kjo.2012.26.3.203.
Expression of the Na(+)-K(+)-2Cl(-)-Cotransporter 2 in the Normal and Pressure-Induced Ischemic Rat Retina
- Affiliations
-
- 1Yang-Ju St. Mary's Eye Clinic, Yangju, Korea.
- 2Department of Ophthalmology and Visual Science, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. mdahn@catholic.ac.kr
- KMID: 1376127
- DOI: http://doi.org/10.3341/kjo.2012.26.3.203
Abstract
- PURPOSE
To evaluate the expression of the Na(+)-K(+)-2Cl(-)-cotransporter 2 (NKCC2) in the ischemic rat retina.
METHODS
Retinal ischemia was induced by pressures 90 to 120 mmHg, above systemic systolic pressure. Immunohistochemistry and western blot analysis were performed.
RESULTS
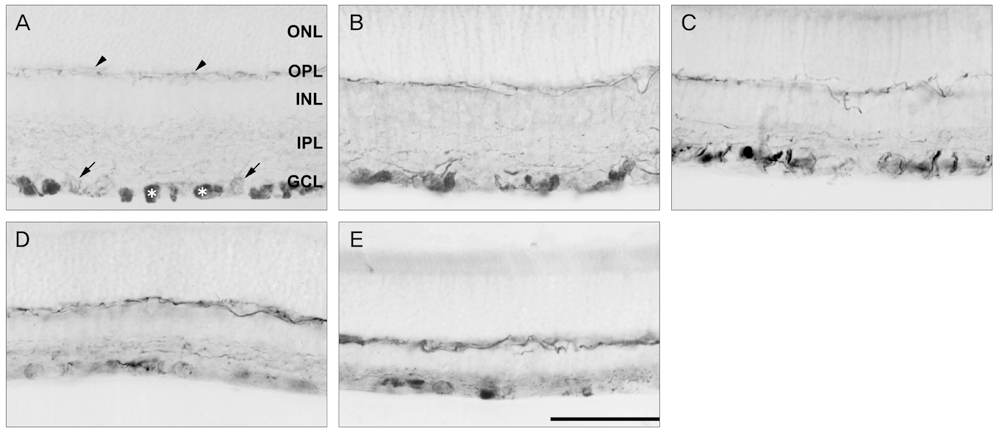
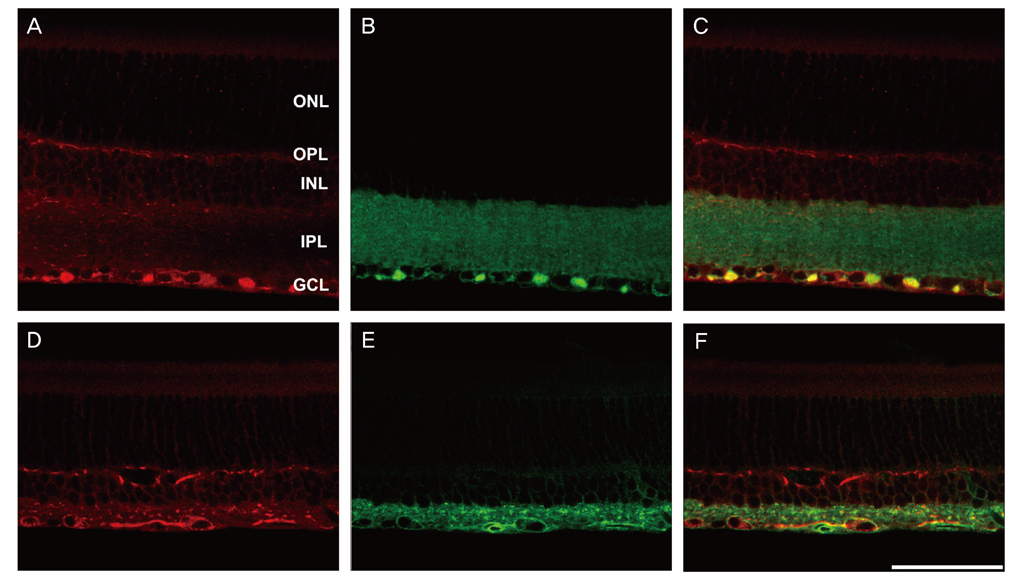
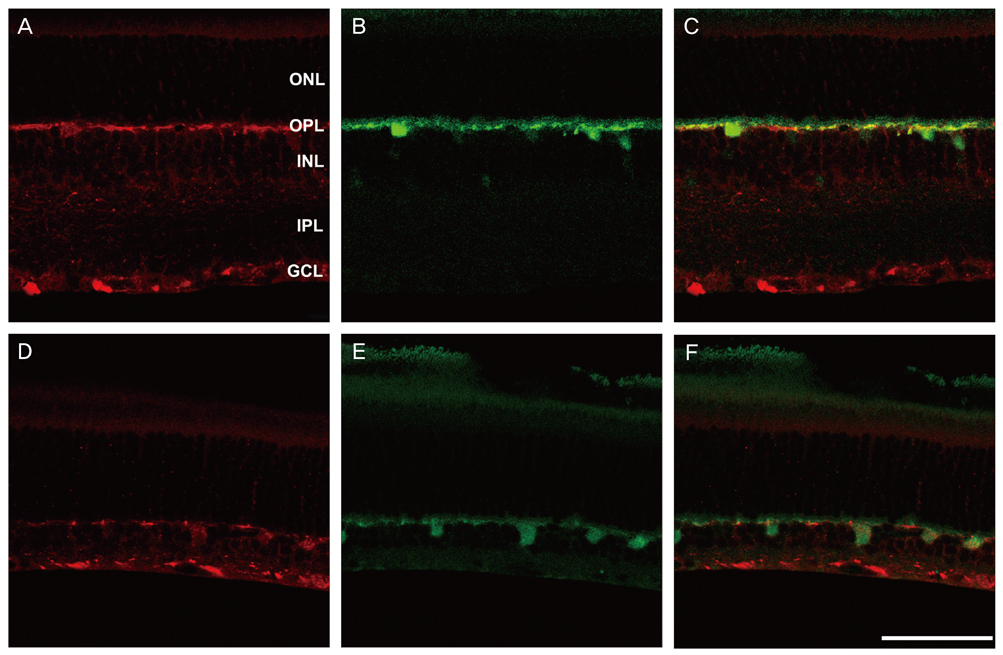
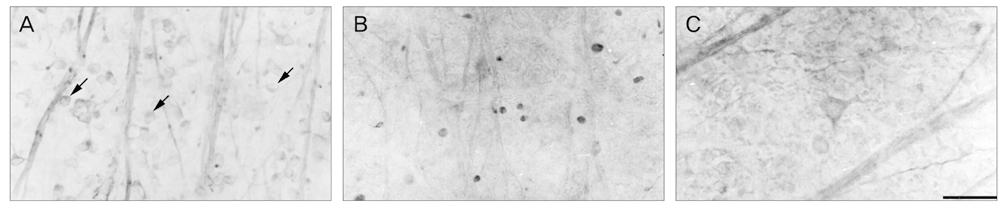
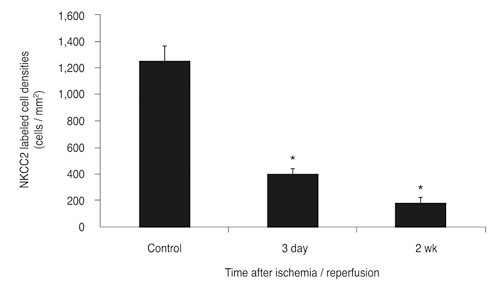
NKCC2 is expressed in the normal retina and its expression is increased by ischemia caused by intraocular pressure elevation. NKCC2 immunoreactivity was observed mainly in axon bundles of ganglion cells and horizontal cell processes in the retina. NKCC2 expression continuously increased with a peak value 3 days (to 415% of normal levels) after ischemic injury, and then gradually decreased to 314% of controls until 2 weeks post injury. The mean density of NKCC2-labeled ganglion cells per mm2 changed from 1,255 +/- 109 in normal retinas to 391 +/- 49 and 185 +/- 37 at 3 days and 2 weeks after ischemia, respectively (p < 0.05), implying cell death of ganglion cells labeled with NKCC2.
CONCLUSIONS
Taken together, these results suggest that NKCC2, which is expressed in retinal ganglion and horizontal cells, may contribute to cell death by ischemic injury in the retina, although the molecular mechanisms involved remain to be clarified.
MeSH Terms
-
Animals
Blotting, Western
Disease Models, Animal
Immunohistochemistry
Intraocular Pressure
Ischemia/etiology/*metabolism
Male
Microscopy, Confocal
Ocular Hypertension/*complications/metabolism/physiopathology
Rats
Rats, Sprague-Dawley
Retinal Diseases/etiology/*metabolism
Retinal Ganglion Cells/*metabolism/pathology
Sodium-Potassium-Chloride Symporters/*biosynthesis
Figure
Reference
-
1. Adorante JS, Miller SS. Potassium-dependent volume regulation in retinal pigment epithelium is mediated by Na,K,Cl cotransport. J Gen Physiol. 1990. 96:1153–1176.2. Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000. 80:211–276.3. Haas M, Forbush B 3rd. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000. 62:515–534.4. Leung S, O'Donnell ME, Martinez A, Palfrey HC. Regulation by nerve growth factor and protein phosphorylation of Na/K/2Cl cotransport and cell volume in PC12 cells. J Biol Chem. 1994. 269:10581–10589.5. Iwamoto LM, Fujiwara N, Nakamura KT, Wada RK. Na-K-2Cl cotransporter inhibition impairs human lung cellular proliferation. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L510–L514.6. Hains BC, Waxman SG. Neuroprotection by sodium channel blockade with phenytoin in an experimental model of glaucoma. Invest Ophthalmol Vis Sci. 2005. 46:4164–4169.7. Kim JA, Kang YY, Lee YS. Activation of Na(+), K(+), Cl(-)-cotransport mediates intracellular Ca(2+) increase and apoptosis induced by Pinacidil in HepG2 human hepatoblastoma cells. Biochem Biophys Res Commun. 2001. 281:511–519.8. Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005. 27:280–286.9. Thomas R, Salter MG, Wilke S, et al. Acute ischemic injury of astrocytes is mediated by Na-K-Cl cotransport and not Ca2+ influx at a key point in white matter development. J Neuropathol Exp Neurol. 2004. 63:856–871.10. Ramasamy R, Payne JA, Whang J, et al. Protection of ischemic myocardium in diabetics by inhibition of electroneutral Na+-K+-2Cl- cotransporter. Am J Physiol Heart Circ Physiol. 2001. 281:H515–H522.11. Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985. 5:1483–1489.12. Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998. 18:3767–3778.13. Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000. 20:7657–7663.14. Lafuente MP, Villegas-Perez MP, Selles-Navarro I, et al. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002. 109:157–168.15. Lafuente MP, Villegas-Perez MP, Sobrado-Calvo P, et al. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2001. 42:2074–2084.16. Lafuente MP, Villegas-Perez MP, Mayor S, et al. Neuroprotective effects of brimonidine against transient ischemia-induced retinal ganglion cell death: a dose response in vivo study. Exp Eye Res. 2002. 74:181–189.17. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997. 37:3483–3493.18. Osborne NN, Ugarte M, Chao M, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999. 43:Suppl 1. S102–S128.19. Osborne NN, Wood JP, Chidlow G. Invited review: neuroprotective properties of certain beta-adrenoceptor antagonists used for the treatment of glaucoma. J Ocul Pharmacol Ther. 2005. 21:175–181.20. Dong CJ, Hare WA. Contribution to ischemic injury of rat optic nerves by intracellular sodium overload. Doc Ophthalmol. 2005. 110:15–23.21. Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006. 31:265–271.22. Gwon JS, Kim IB, Lee MY, et al. Expression of clusterin in Müller cells of the rat retina after pressure-induced ischemia. Glia. 2004. 47:35–45.23. Dijk F, van Leeuwen S, Kamphuis W. Differential effects of ischemia/reperfusion on amacrine cell subtype-specific transcript levels in the rat retina. Brain Res. 2004. 1026:194–204.24. Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, et al. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996. 37:2002–2014.25. Siliprandi R, Canella R, Carmignoto G, et al. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992. 8:567–573.26. Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991. 203:138–147.27. Huang SY, Liang PJ. Ca2+-permeable and Ca2+-impermeable AMPA receptors coexist on horizontal cells. Neuroreport. 2005. 16:263–266.28. Chun MH, Kim IB, Ju WK, et al. Horizontal cells of the rat retina are resistant to degenerative processes induced by ischemia-reperfusion. Neurosci Lett. 1999. 260:125–128.29. Wassle H, Peichl L, Airaksinen MS, Meyer M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998. 292:211–218.30. Kobayashi M, Kuroiwa T, Shimokawa R, et al. Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol. 2000. 44:235–244.31. Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005. 46:4747–4753.32. Massey SC, O'Brien JJ, Trexler EB, et al. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes. 2003. 10:425–430.33. Garcia-Dorado D, Ruiz-Meana M, Padilla F, et al. Gap junction-mediated intercellular communication in ischemic preconditioning. Cardiovasc Res. 2002. 55:456–465.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Expression of Renal Aquaporin-2 and Na-K-2Cl Cotransporter in Bilateral Ureteral Obstruction (BUO) Rat after Release of BUO and the Effect of Antidiuretic Hormone
- Increased Expression of Sodium Transporters in Rats Chronically Inhibited of Nitric Oxide Synthesis
- Altered Regulation of type 3 Na+/H+ exchanger, type 1 Na+/HCO3- cotransporter, and Na+,K+-ATPase in the Kidney of Rats with Experimental Rhabdomyolysis
- Localization of E-cadherin Along the Nephron Segments in the Rat Kidney
- Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina