Anat Cell Biol.
2010 Mar;43(1):44-53. 10.5115/acb.2010.43.1.44.
Recovered changes in the spleen by agmatine treatment after transient cerebral ischemia
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Health Sciences University of Mongolia, Ulaanbaatar, Mongolia.
- KMID: 2168896
- DOI: http://doi.org/10.5115/acb.2010.43.1.44
Abstract
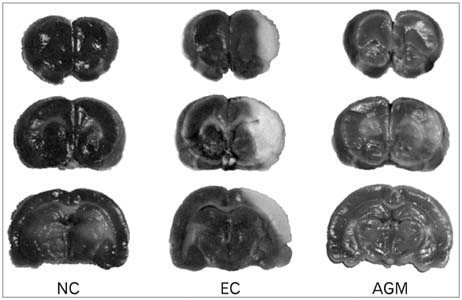
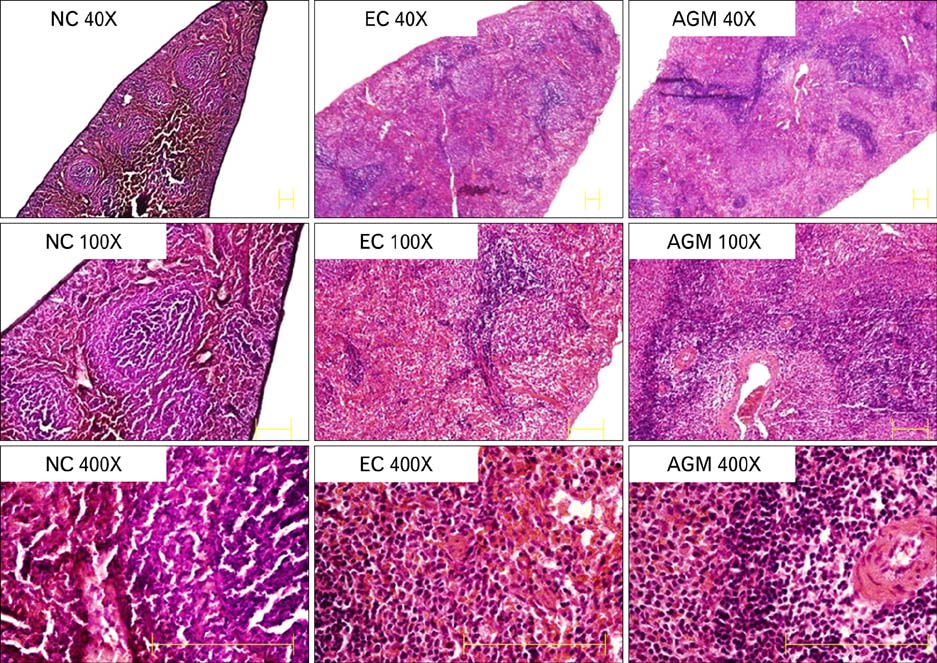
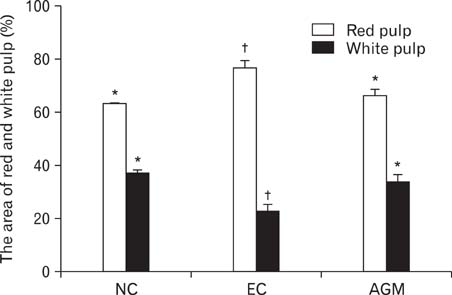
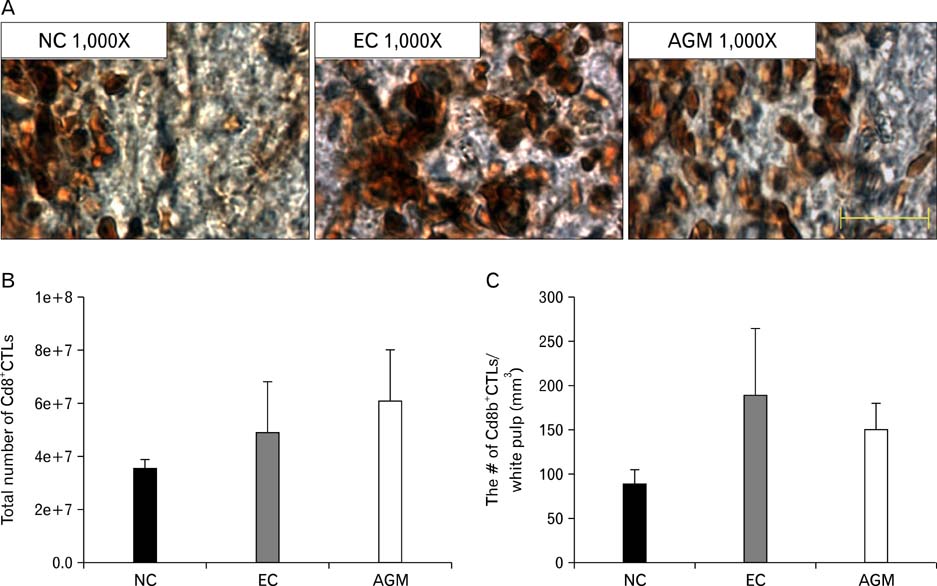
- Stroke or cerebrovascular injury is the leading cause of disability and the third leading cause of deaths worldwide. After the initial ischemic injury, sympathetic signals are transmitted to the spleen and a compromised blood-brain barrier, coupled with expression of adhesion molecules by the vascular endothelial cells permits an influx of peripheral immune cells. This influx of peripheral immune cells into the brain exacerbates the local brain inflammatory response, leading to enhanced neurodegeneration. Agmatine is a primary amine formed by decarboxylation of L-arginine synthesized in the mammalian brain. In this study, we determined the effect of agmatine on the immune response in the spleen after transient cerebral ischemia. Twenty-three hours after transient cerebral ischemia, the white pulp area was reduced and the number of CD11b+ macrophages and CD4+CD25+ regulatory T cells (T reg cells) were increased in the spleens in the experimental group as a result of alteration of the immune response in the spleen, as regulated by inflammatory cytokines. In the agmatine treatment group (100 mg/kg IP), the contraction of white pulp was diminished and the number of CD11b+ macrophages and CD4+CD25+T reg cells were decreased. Twenty-three hours after transient cerebral ischemia, the brain infarction area was significantly reduced (5.51+/-1.63% of the whole brain) in the agmatine treatment group compared to 15.02+/-4.28% of the whole brain in the experimental control group. These results suggest that agmatine treatment can reduce brain infarction through minimizing neuroinflammation and can lessen the danger of post-stroke infection from depression of the immune system after stroke.
MeSH Terms
Figure
Reference
-
1. Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 2007. 6th Ed. 192–196. 252–253.2. Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995. 69:285–287.3. Batbaatar G. Basic immunology. 2007. 4th Ed. 67–75.4. Emsley HC, Smith CJ, Gavin CM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003. 139:93–101.5. Garcia Y, Breen A, Burugapalli K, Dockery P, Pandit A. Stereological methods to assess tissue response for tissue-engineered scaffolds. Biomaterials. 2007. 28:175–186.6. Gendron A, Teitelbaum J, Cossette C, et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002. 955:85–97.7. Kim JH, Lee YW, Kim JY, Lee WT, Park KA, Lee JE. The effect of agmatine on expression of MMP2 and MMP9 in Cerebral ischemia. Korean J Anat. 2008. 41:97–104.8. Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004. 189:122–130.9. Kim SY, Choi KC, Chang MS, et al. The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J Neurosci. 2006. 26:4567–4576.10. Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic re ceptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001. 53:487–525.11. Lee JE, Yenari MA, Sun GH, et al. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001. 170:129–139.12. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001. 193:1295–1302.13. Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994. 263:966–969.14. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006. 147:Suppl 1. S232–S240.15. Molderings GJ, Bonisch H, Hammermann R, Göthert M, Bruss M. Noradrenaline release-inhibiting receptors on PC12 cells devoid of alpha (2(-)) and CB(1) receptors: similarities to presynaptic imidazoline and edg receptors. Neurochem Int. 2002. 40:157–167.16. Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006. 26:654–665.17. Piletz JE, Chikkala DN, Ernsberger P. Comparison of the properties of agmatine and endogenous clonidine-displacing substance at imidazoline and alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1995. 272:581–587.18. Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003. 198:725–736.19. Regunathan S, Piletz JE. Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci. 2003. 1009:20–29.20. Reynolds IJ. Arcaine uncovers dual interactions of polyamines with the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1990. 255:1001–1007.21. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25) Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.22. Stewart IB, McKenzie DC. The human spleen during physiological stress. Sports Med. 2002. 32:361–369.23. Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998. 10:1969–1980.24. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998. 188:287–296.25. Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006. 199:191–200.26. West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993. 14:275–285.27. Wolf BC, Neiman RS. Hypothesis: splenic filtration and the pathogenesis of extramedullary hematopoiesis in agnogenic myeloid metaplasia. Hematol Pathol. 1987. 1:77–80.28. Yang XC, Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther. 1999. 288:544–549.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Agmatine on Expression of MMP2 and MMP9 in Cerebral Ischemia
- Quantitative Analysis of Agmatine by HPLC in Ischemic Brain
- Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain
- Agmatine Attenuates Nitric Oxide Synthesis and Protects ER-structure from Global Cerebral Ischemia in Rats
- Endogenous Agmatine Induced by Ischemic Preconditioning Regulates Ischemic Tolerance Following Cerebral Ischemia