Anat Cell Biol.
2010 Sep;43(3):230-240. 10.5115/acb.2010.43.3.230.
Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea.
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2168878
- DOI: http://doi.org/10.5115/acb.2010.43.3.230
Abstract
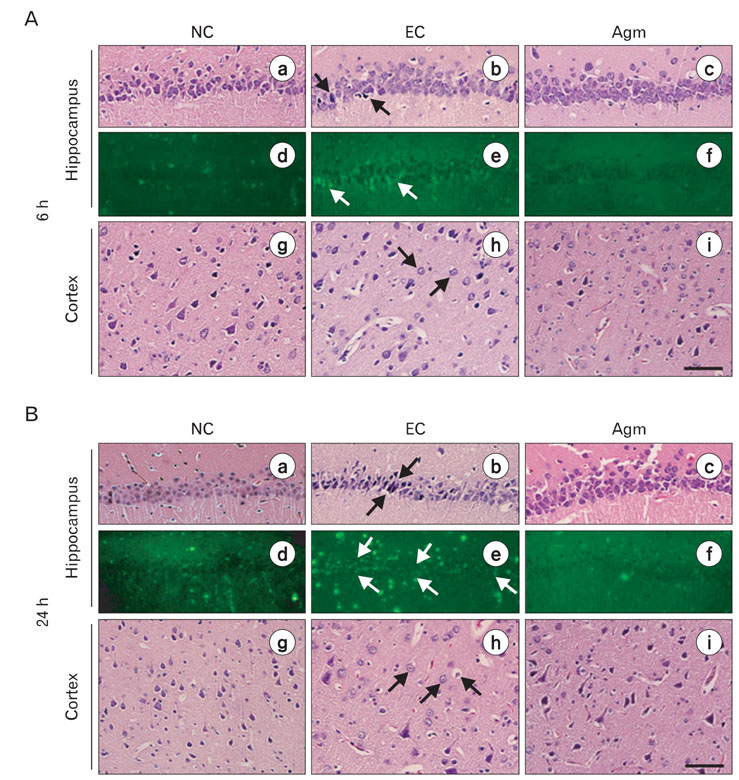
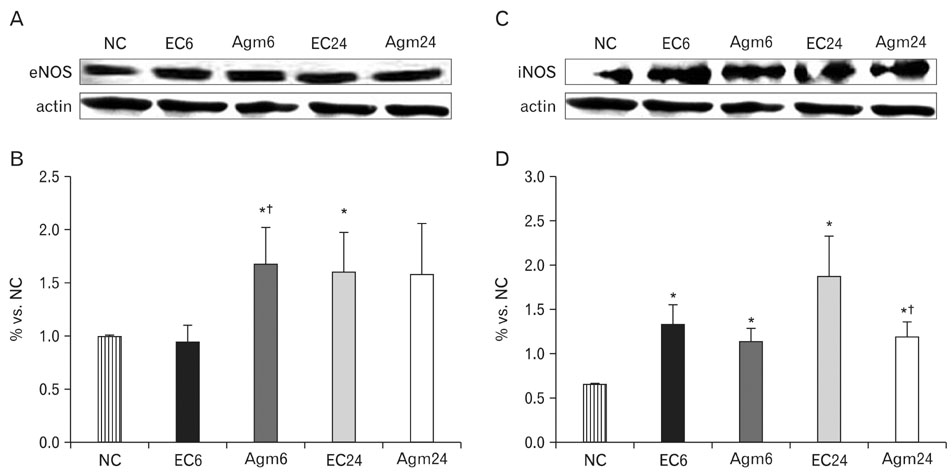
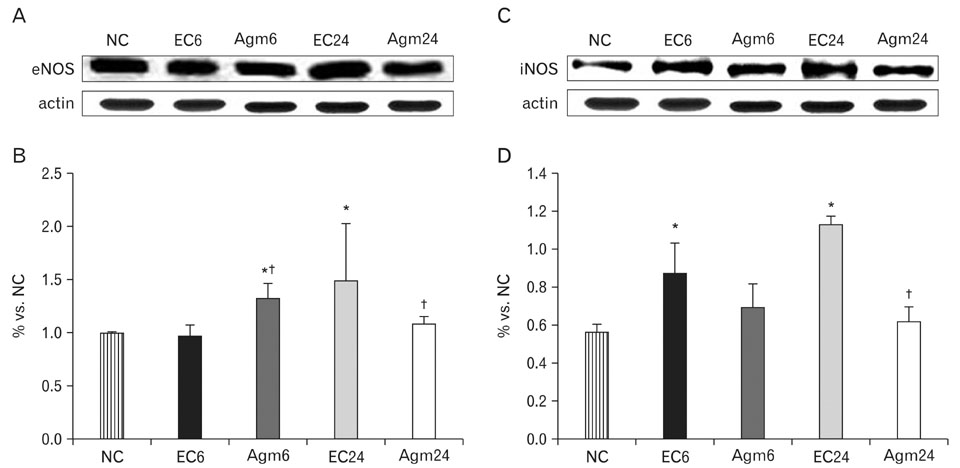
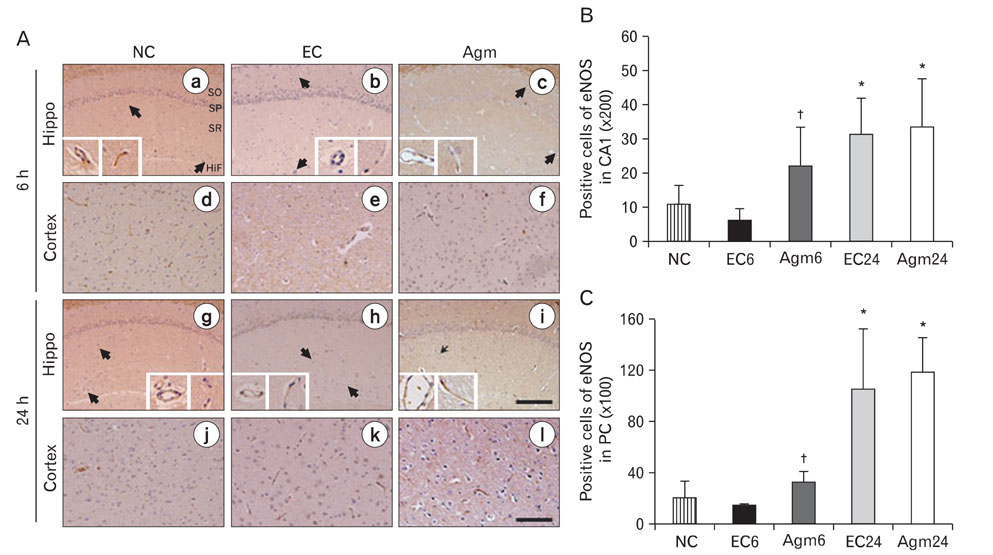
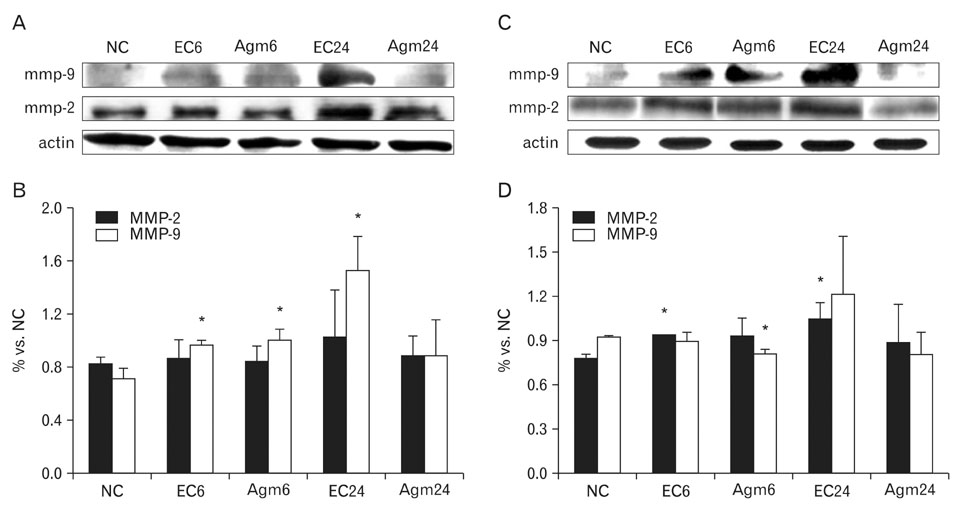
- Nitric oxide (NO) production by endothelial nitric oxide synthase (eNOS) plays a protective role in cerebral ischemia by maintaining vascular permeability, whereas NO derived from neuronal and inducible NOS is neurotoxic and can participate in neuronal damage occurring in ischemia. Matrix metalloproteinases (MMPs) are up-regulated by ischemic injury and degrade the basement membrane if brain vessels to promote cell death and tissue injury. We previously reported that agmatine, synthesized from L-arginine by arginine decarboxylase (ADC) which is expressed in endothelial cells, has shown a direct increased eNOS expression and decreased MMPs expression in bEnd3 cells. But, there are few reports about the regulation of eNOS by agmatine in ischemic animal model. In the present study, we examined the expression of eNOS and MMPs by agmatine treatment after transient global ischemia in vivo. Global ischemia was induced with four vessel occlusion (4-VO) and agmatine (100 mg/kg) was administered intraperitoneally at the onset of reperfusion. The animals were euthanized at 6 and 24 hours after global ischemia and prepared for other analysis. Global ischemia led severe neuronal damage in the rat hippocampus and cerebral cortex, but agmatine treatment protected neurons from ischemic injury. Moreover, the level and expression of eNOS was increased by agmatine treatment, whereas inducible NOS (iNOS) and MMP-9 protein expressions were decreased in the brain. These results suggest that agmatine protects microvessels in the brain by activation eNOS as well as reduces extracellular matrix degradation during the early phase of ischemic insult.
MeSH Terms
-
Agmatine
Animals
Arginine
Basement Membrane
Brain
Brain Ischemia
Capillary Permeability
Carboxy-Lyases
Cell Death
Cerebral Cortex
Endothelial Cells
Extracellular Matrix
Glycosaminoglycans
Hippocampus
Ischemia
Matrix Metalloproteinases
Microvessels
Models, Animal
Neurons
Nitric Oxide
Nitric Oxide Synthase Type III
Rats
Reperfusion
Agmatine
Arginine
Carboxy-Lyases
Glycosaminoglycans
Matrix Metalloproteinases
Nitric Oxide
Nitric Oxide Synthase Type III
Figure
Cited by 2 articles
-
Suppression of MicroRNA let-7a Expression by Agmatine Regulates Neural Stem Cell Differentiation
Juhyun Song, Yumi Oh, Jong Youl Kim, Kyoung Joo Cho, Jong Eun Lee
Yonsei Med J. 2016;57(6):1461-1467. doi: 10.3349/ymj.2016.57.6.1461.Role of agmatine in the application of neural progenitor cell in central nervous system diseases: therapeutic potentials and effects
Renée Kosonen, Sumit Barua, Jong Youl Kim, Jong Eun Lee
Anat Cell Biol. 2021;54(2):143-151. doi: 10.5115/acb.21.089.
Reference
-
1. Abe K, Abe Y, Saito H. Agmatine suppresses nitric oxide production in microglia. Brain Res. 2000. 872:141–148.2. Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001a. 12:3003–3007.3. Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001b. 21:7724–7732.4. Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003. 34:1299–1303.5. Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995. 69:285–287.6. Blantz RC, Satriano J, Gabbai F, Kelly C. Biological effects of arginine metabolites. Acta Physiol Scand. 2000. 168:21–25.7. Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience. 2009. 159:744–750.8. Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2002. 52:606–611.9. Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002. 297:1186–1190.10. Huang PL. Nitric oxide and cerebral ischemic preconditioning. Cell Calcium. 2004. 36:323–329.11. Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997. 20:132–139.12. Joshi MS, Ferguson TB Jr, Johnson FK, Johnson RA, Parthasarathy S, Lancaster JR Jr. Receptor-mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc Natl Acad Sci U S A. 2007. 104:9982–9987.13. Jung HJ, Yang MZ, Kwon KH, et al. Endogenous agmatine inhibits cerebral vascular matrix metallo-proteinases expression by regulating activating transcription factor 3 and endothelial nitric oxide synthesis. Curr Neurovasc Res. 2010. 7:201–212.14. Katsuta K, Umemura K, Ueyama N, Matsuoka N. Pharmacological evidence for a correlation between hippocampal CA1 cell damage and hyperlocomotion following global cerebral ischemia in gerbils. Eur J Pharmacol. 2003. 467:103–109.15. Kim DJ, Kim DI, Lee SK, et al. Protective effect of agmatine on a reperfusion model after transient cerebral ischemia: temporal evolution on perfusion MR imaging and histopathologic findings. AJNR Am J Neuroradiol. 2006. 27:780–785.16. Kim JH, Lee YW, Kim JY, Lee WT, Park KA, Lee JE. The effect of agmatine on expression of MMP2 and MMP9 in Cerebral ischemia. Korean J Anat. 2008. 41:97–104.17. Kim JH, Lee YW, Park KA, Lee WT, Lee JE. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J Cereb Blood Flow Metab. 2010. 30:943–949.18. Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004. 189:122–130.19. Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci Lett. 1997. 224:17–20.20. Lee JE, Yenari MA, Sun GH, et al. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001. 170:129–139.21. Lee WT, Hong S, Yoon SH, et al. Neuroprotective effects of agmatine on oxygen-glucose deprived primary-cultured astrocytes and nuclear translocation of nuclear factor-kappa B. Brain Res. 2009. 1281:64–70.22. Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994. 263:966–969.23. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003. 4:399–415.24. Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002. 69:1–9.25. Marks KA, Mallard CE, Roberts I, Williams CE, Gluckman PD, Edwards AD. Nitric oxide synthase inhibition attenuates delayed vasodilation and increases injury after cerebral ischemia in fetal sheep. Pediatr Res. 1996. 40:185–191.26. Moro MA, Cárdenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004. 36:265–275.27. Morrissey JJ, Klahr S. Agmatine activation of nitric oxide synthase in endothelial cells. Proc Assoc Am Physicians. 1997. 109:51–57.28. Mun CH, Kim JH, Park KA, Lee WT, Baik JH, Lee JE. Agmatine attenuates nitric oxide synthesis and protects ER-structure from global cerebral ischemia in rats. Korean J Anat. 2009. 42:149–160.29. Mun CH, Lee WT, Park KA, Lee JE. Agmatine reduced the expressions of nitric oxide synthase and peroxynitrite formation in rat cerebral cortex after transient global cerebral ischemia. Neural Regen Res. In Press.30. Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998. 152:729–741.31. Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int. 2006. 49:170–182.32. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1998. 4th ed. New York: Academic Press.33. Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979. 10:267–272.34. Raasch W, Schäfer U, Chun J, Dominiak P. Biological significance of agmatine, an endogenous ligand at imidazoline binding sites. Br J Pharmacol. 2001. 133:755–780.35. Raghavan SA, Dikshit M. Vascular regulation by the L-arginine metabolites, nitric oxide and agmatine. Pharmacol Res. 2004. 49:397–414.36. Rivera S, Ogier C, Jourquin J, et al. Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity after global forebrain ischemia. Eur J Neurosci. 2002. 15:19–32.37. Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002. 39:279–291.38. Santhanam AV, Viswanathan S, Dikshit M. Activation of protein kinase B/Akt and endothelial nitric oxide synthase mediates agmatine-induced endothelium-dependent relaxation. Eur J Pharmacol. 2007. 572:189–196.39. Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996. 384:123–124.40. Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002. 33:2704–2710.41. Wang X, Feuerstein GZ. Role of immune and inflammatory mediators in CNS injury. Drug News Perspect. 2000. 13:133–140.42. Yang MZ, Mun CH, Choi YJ, et al. Agmatine inhibits matrix metalloproteinase-9 via endothelial nitric oxide synthase in cerebral endothelial cells. Neurol Res. 2007. 29:749–754.43. Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998. 21:75–80.44. Zalewska T, Ziemka-Nacz M, Sarnowska A, Domańska-Janik K. Involvement of MMPs in delayed neuronal death after global ischemia. Acta Neurobiol Exp (Wars). 2002. 62:53–61.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Agmatine Attenuates Nitric Oxide Synthesis and Protects ER-structure from Global Cerebral Ischemia in Rats
- Nitric Oxide Synthase Inhibition Alters Extracellular Glutamate Concentration after Global Cerebral Ischemia
- Endogenous Agmatine Induced by Ischemic Preconditioning Regulates Ischemic Tolerance Following Cerebral Ischemia
- The Effect of Agmatine on Expression of MMP2 and MMP9 in Cerebral Ischemia
- Effect of PAF antagonists on the nitric oxide synthesis in ischemic cerebral cortex