Anat Cell Biol.
2010 Mar;43(1):25-35. 10.5115/acb.2010.43.1.25.
Vitamin C acts indirectly to modulate isotype switching in mouse B cells
- Affiliations
-
- 1Department of Anatomy and Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul, Korea. hyi830@snu.ac.kr
- 2Department of Rehabilitation Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2168894
- DOI: http://doi.org/10.5115/acb.2010.43.1.25
Abstract
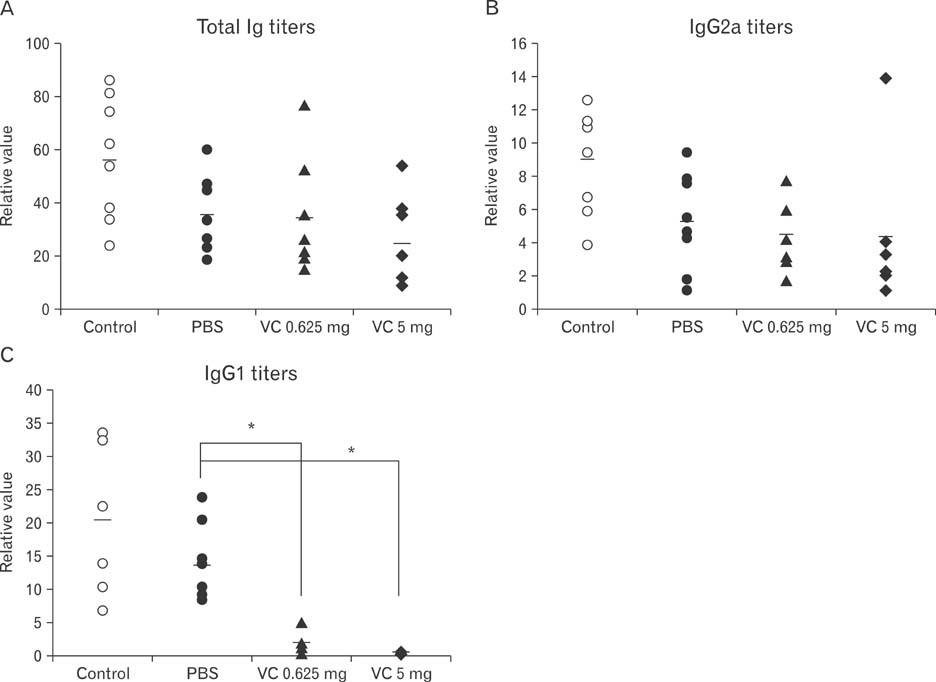
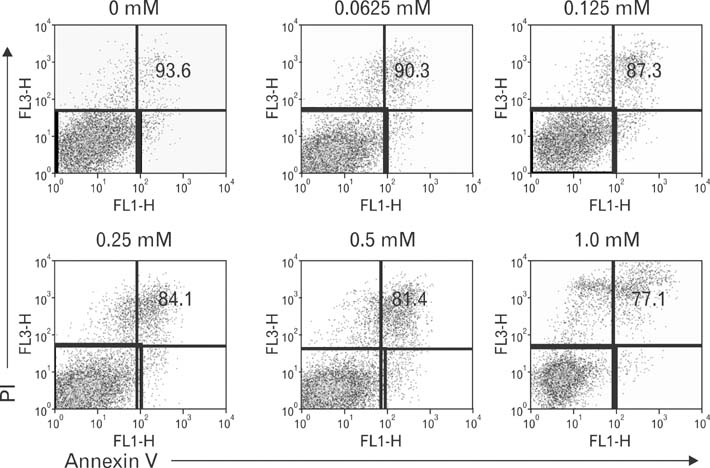
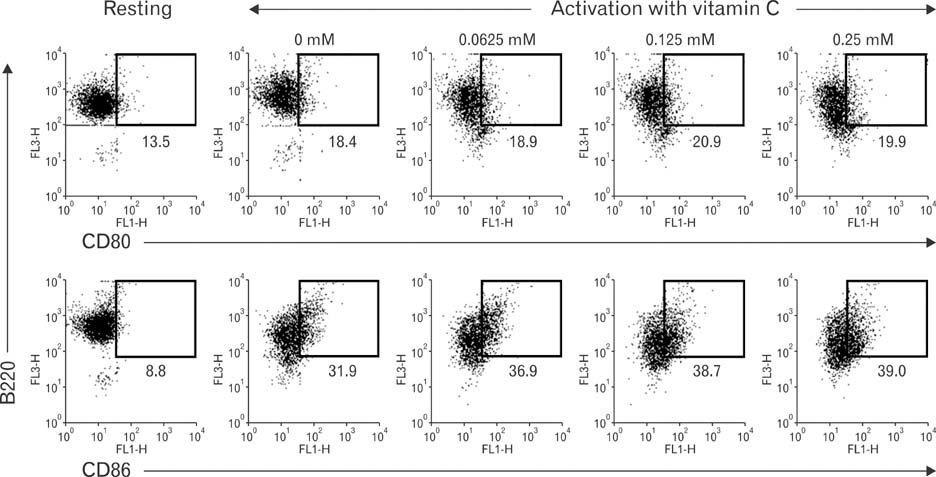
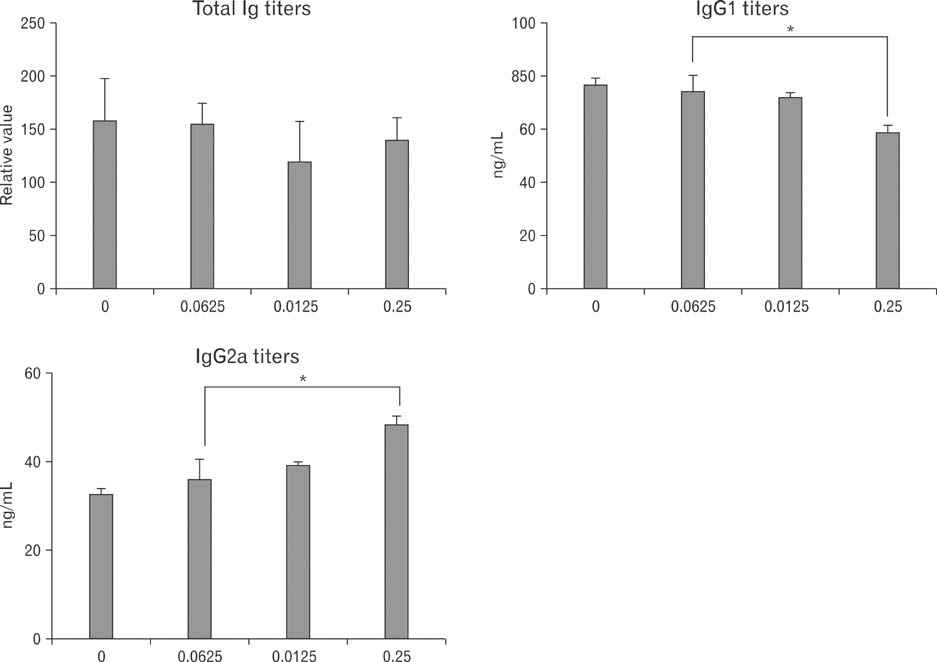
- Vitamin C, one of essential micronutrients, has been reported to modulate the humoral immune responses in some mammals. We investigated whether vitamin C might modulate this response in mice by directly affecting B cells. Splenic B cells were isolated and activated by CD40- and B cell receptor-ligation in vitro. The cells were cultured with a pretreatment of vitamin C from 0 to 1 mM of concentrations. Vitamin C slightly increased apoptosis of B cells dose-dependently and behaved as an antioxidant. We found that in vivo administration of vitamin C by intraperitoneal injection affected isotype switching as previously reported: the titer of antigen-specific IgG1 antibody was decreased, while that of IgG2a was unaffected. Somewhat different from those observed in vivo, in vitro exposure to vitamin C slightly decreased isotype switching to IgG1 and increased isotype switching to IgG2a. Pretreatment with vitamin C in the safe range did not affect either proliferation of cultured B cells or the expression of CD80 and CD86 in those cells. Taken together, in vivo results suggest that vitamin C acts to modulate isotype switching in the mouse. However, because of our in vitro results, we suggest that the modulation exerted by vitamin C in vivo is by indirectly affecting B cells, perhaps by directly influencing other immune cells such as dendritic cells.
MeSH Terms
Figure
Reference
-
1. Anderson R, Dittrich OC. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979. 56:476–480.2. Anderson R, Hay I, van Wyk H, Oosthuizen R, Theron A. The effect of ascorbate on cellular humoral immunity in asthmatic children. S Afr Med J. 1980. 58:974–977.3. Basso K, Klein U, Niu H, et al. Tracking CD40 signaling during germinal center development. Blood. 2004. 104:4088–4096.4. Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000. 176:171–180.5. Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol Lett. 2006. 104:70–75.6. Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001. 24:97–109.7. Blumenthal RD, Lew W, Reising A, et al. Anti-oxidant vitamins reduce normal tissue toxicity induced by radio-immunotherapy. Int J Cancer. 2000. 86:276–280.8. Bowie AG, O'Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000. 165:7180–7188.9. Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006. 25:6731–6748.10. Caamaño JH, Rizzo CA, Durham SK, et al. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998. 187:185–196.11. Clément MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001. 3:157–163.12. Cummins KA, Brunner CJ. Dietary ascorbic acid and immune response in dairy calves. J Dairy Sci. 1989. 72:129–134.13. De Tullio MC. Asard H, May JM, Smirnoff N, editors. How does ascorbic acid prevent scurvy? A survey of the nonantioxidant functions of vitamin C. Vitamin C Function and Biochemistry in Animals and Plants. 2002. BIOS Scientific Publisher;159–171.14. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002. 82:47–95.15. Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005. 39:671–686.16. Dubick MA, Carden SC, Jordan BS, Langlinais PC, Mozingo DW. Indices of antioxidant status in rats subjected to wood smoke inhalation and/or thermal injury. Toxicology. 2002. 176:145–157.17. Dubick MA, Critchfield JW, Last JA, Cross CE, Rucker RB. Ascorbic acid turnover in the mouse following acute ozone exposure. Toxicology. 1983. 27:301–313.18. Gong J, Chen SS. Polyphenolic antioxidants inhibit peptide presentation by antigen-presenting cells. Int Immunopharmacol. 2003. 3:1841–1852.19. Guaiquil VH, Vera JC, Golde DW. Mechanism of vitamin C inhibition of cell death inducedby oxidative stress in glutathione-depleted HL-60 cells. J Biol Chem. 2001. 276:40955–40961.20. Han SS, Kim K, Hahm ER, et al. L-ascorbic acid represses constitutive activation of NF-kappaB and COX-2 expression in human acute myeloid leukemia, HL-60. J Cell Biochem. 2004. 93:257–270.21. Hasbold J, Hong JS, Kehry MR, Hodgkin PD. Integrating signals from IFN-gamma and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J Immunol. 1999. 163:4175–4181.22. Haxhinasto SA, Bishop GA. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J Biol Chem. 2004. 279:2575–2582.23. Hoffer LJ, Levine M, Assouline S, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008. 19:1969–1974.24. Iijima N, Yanagawa Y, Iwabuchi K, Onoe K. Selective regulation of CD40 expression in murine dendritic cells by thiol antioxidants. Immunology. 2003. 110:197–205.25. Ito K, Takubo K, Arai F, et al. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007. 178:103–110.26. Jeannin P, Delneste Y, Lecoanet-Henchoz S, et al. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. J Exp Med. 1995. 182:1785–1792.27. Kuo SM, MacLean ME, McCormick K, Wilson JX. Gender and sodium-ascorbate transporter isoforms determine ascorbate concentrations in mice. J Nutr. 2004. 134:2216–2221.28. Lee CW, Wang XD, Chien KL, et al. Vitamin C supplementation does not protect L-gulono-gamma-lactone oxidase-deficient mice from Helicobacter pylori-induced gastritis and gastric premalignancy. Int J Cancer. 2008. 122:1068–1076.29. Lee JR. Reactive oxygen species play roles on B cell surface receptor CD40-mediated proximal and distal signaling events: effects of an antioxidant, N-acetyl-L-cysteine treatment. Mol Cell Biochem. 2003. 252:1–7.30. Lee JS, Kim SG, Kim HK, et al. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J Cell Physiol. 2007. 210:385–397.31. Li MJ, Peakman MC, Golub EI, et al. Rad51 expression and localization in B cells carrying out class switch recombination. Proc Natl Acad Sci USA. 1996. 93:10222–10227.32. Long KZ, Santos JI. Vitamins and the regulation of the immune response. Pediatr Infect Dis J. 1999. 18:283–290.33. Maemura K, Zheng Q, Wada T, et al. Reactive oxygen species are essential mediators in antigen presentation by Kupffer cells. Immunol Cell Biol. 2005. 83:336–343.34. Maeng HG, Lim H, Jeong YJ, et al. Vitamin C enters mouse T cells as dehydroascorbic acid in vitro and does not recapitulate in vivo vitamin C effects. Immunobiology. 2009. 214:311–320.35. Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009. 157:1097–1110.36. Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002. 23:31–39.37. Noh K, Kim HG, Shin YA, et al. The effects of high-dose vitamin C administration on the cell-mediated immune response in mice. Immune Netw. 2003. 3:211–218.38. Noh K, Lim H, Moon SK, et al. Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005. 98:63–72.39. Nouri-Shirazi M, Guinet E. Direct and indirect cross-tolerance of alloreactive T cells by dendritic cells retained in the immature stage. Transplantation. 2002. 74:1035–1044.40. Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006. 174:937–942.41. Pan-Hammarström Q, Zhao Y, Hammarström L. Class switch recombination: a comparison between mouse and human. Adv Immunol. 2007. 93:1–61.42. Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006. 8:1791–1806.43. Patak P, Willenberg HS, Bornstein SR. Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocr Res. 2004. 30:871–875.44. Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006. 90:82–90.45. Takatsu K. Cytokines involved in B-cell differentiation and their sites of action. Proc Soc Exp Biol Med. 1997. 215:121–133.46. Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol. 1999. 162:2569–2574.47. Wan CP, Myung E, Lau BH. An automated micro-fluorometric assay for monitoring oxidative burst activity of phagocytes. J Immunol Methods. 1993. 159:131–138.48. Weil R, Israël A. T-cell-receptor- and B-cell-receptor-mediated activation of NF-kappaB in lymphocytes. Curr Opin Immunol. 2004. 16:374–381.49. Yanagihara Y, Basaki Y, Kajiwara K, Ikizawa K. A thiol antioxidant regulates IgE isotype switching by inhibiting activation of nuclear factor-kappaB. J Allergy Clin Immunol. 1997. 100:S33–S38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Mouse B Lymphoma Cells (CH12F3-2A) for the Study of IgA Isotype Switching
- Alum Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype
- Lactoferrin Combined with Retinoic Acid Stimulates B1 Cells to Express IgA Isotype and Gut-homing Molecules
- T Cell Dependent Antigen-Induced Immunoglobulin Isotype Swiching and Diifferentiation of Lymph Node
- Immunoglobulin Isotype Switching in a Plasma Cell Myeloma Patient Treated with High-dose Chemotherapy and Hematopoietic Stem Cell Transplantation