Immune Netw.
2015 Feb;15(1):37-43. 10.4110/in.2015.15.1.37.
Lactoferrin Combined with Retinoic Acid Stimulates B1 Cells to Express IgA Isotype and Gut-homing Molecules
- Affiliations
-
- 1Department of Molecular Bioscience, School of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea. phkim@kangwon.ac.kr
- 2Department of Microbiology, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- 3Department of Animal Life and Environmental Science, College of Agriculture and Life Science, Hankyong National University, Anseong 456-749, Korea.
- KMID: 2150828
- DOI: http://doi.org/10.4110/in.2015.15.1.37
Abstract
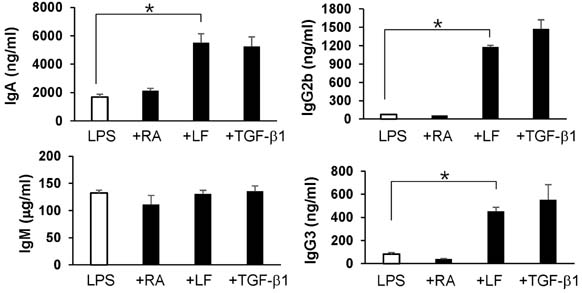
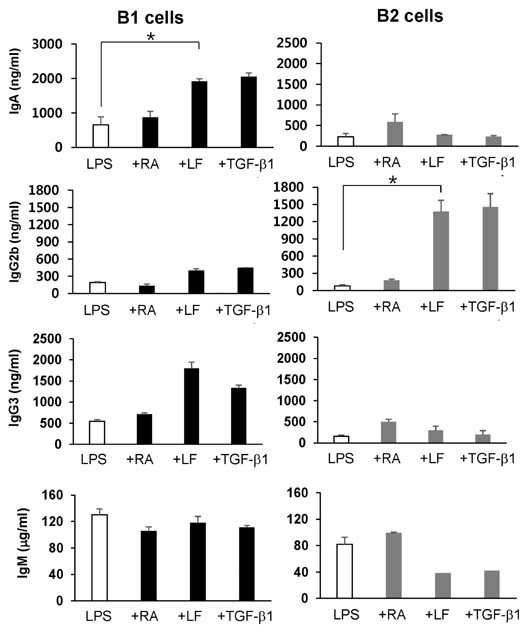
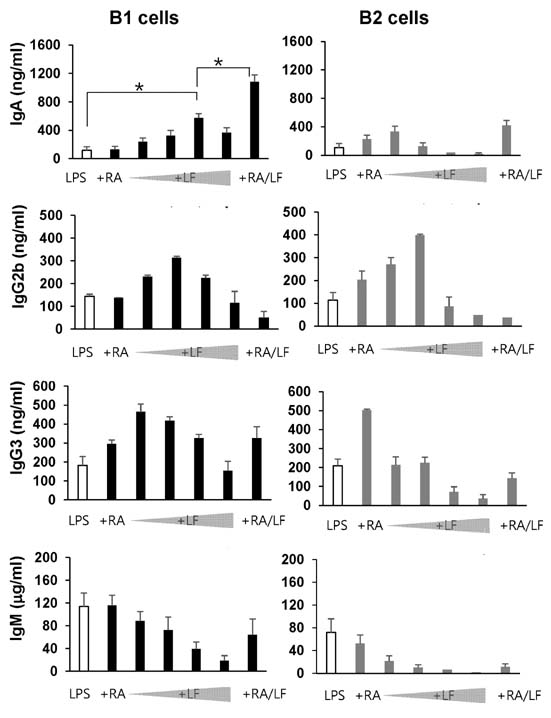
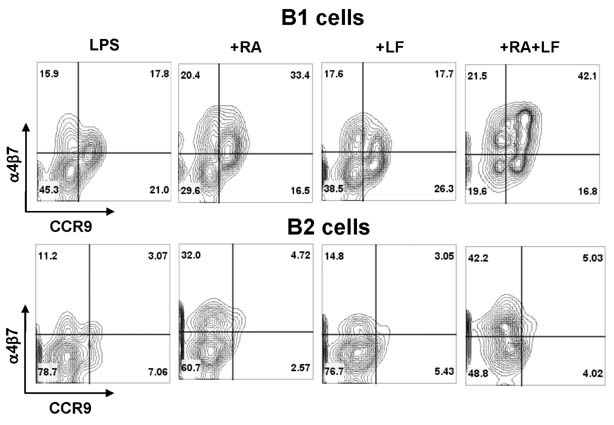
- It is well established that TGF-beta1 and retinoic acid (RA) cause IgA isotype switching in mice. We recently found that lactoferrin (LF) also has an activity of IgA isotype switching in spleen B cells. The present study explored the effect of LF on the Ig production by mouse peritoneal B cells. LF, like TGF-beta1, substantially increased IgA production in peritoneal B1 cells but little in peritoneal B2 cells. In contrast, LF increased IgG2b production in peritoneal B2 cells much more strongly than in peritoneal B1 cells. LF in combination with RA further enhanced the IgA production and, interestingly, this enhancement was restricted to IgA isotype and B1 cells. Similarly, the combination of the two molecules also led to expression of gut homing molecules alpha4beta7 and CCR9 on peritoneal B1 cells, but not on peritoneal B2 cells. Thus, these results indicate that LF and RA can contribute to gut IgA response through stimulating IgA isotype switching and expression of gut-homing molecules in peritoneal B1 cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Control of Innate and Adaptive Lymphocytes by the RAR-Retinoic Acid Axis
Chang H. Kim
Immune Netw. 2018;18(1):. doi: 10.4110/in.2018.18.e1.
Reference
-
1. Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983; 157:202–218.
Article2. Gray D, MacLennan IC, Bazin H, Khan M. Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur J Immunol. 1982; 12:564–569.3. Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989; 19:2163–2166.
Article4. Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013; 13:118–132.
Article5. Marcos MA, Huetz F, Pereira P, Andreu JL, Martinez A, Coutinho A. Further evidence for coelomic-associated B lymphocytes. Eur J Immunol. 1989; 19:2031–2035.
Article6. Kroese FG, Ammerlaan WA, Deenen GJ. Location and function of B-cell lineages. Ann N Y Acad Sci. 1992; 651:44–58.7. Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991; 21:2951–2962.
Article8. Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992; 13:17–21.
Article9. Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001; 14:617–629.
Article10. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011; 11:34–46.
Article11. Kearney JF. Innate-like B cells. Springer Semin Immunopathol. 2005; 26:377–383.
Article12. Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008; 1:11–22.
Article13. Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010; 28:243–273.
Article14. Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008; 28:740–750.
Article15. Suzuki K, Maruya M, Kawamoto S, Fagarasan S. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol Rev. 2010; 237:180–190.
Article16. Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989; 1:75–84.
Article17. Kroese FG, Butcher EC, Stall AM, Herzenberg LA. A major peritoneal reservoir of precursors for intestinal IgA plasma cells. Immunol Invest. 1989; 18:47–58.
Article18. Bos NA, Bun JC, Popma SH, Cebra ER, Deenen GJ, van der Cammen MJ, Kroese FG, Cebra JJ. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996; 64:616–623.
Article19. Coffman RL, Lebman DA, Shrader B. Transforming growth fact or beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989; 170:1039–1044.
Article20. Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989; 170:1415–1420.
Article21. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990; 145:3773–3778.22. Tokuyama H, Tokuyama Y. Retinoids enhance IgA production by lipopolysaccharide-stimulated murine spleen cells. Cell Immunol. 1993; 150:353–363.
Article23. Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999; 192:41–47.
Article24. Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR, Lee JM, Choe J, Kim PH. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta1 to enhance the overall IgA response. J Leukoc Biol. 2013; 94:325–335.
Article25. Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006; 177:6025–6029.
Article26. Roy B, Brennecke AM, Agarwal S, Krey M, Duber S, Weiss S. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-beta and retinoic acid. PLoS One. 2013; 8:e82121.27. Hart AL, Ng SC, Mann E, Al-Hassi HO, Bernardo D, Knight SC. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm Bowel Dis. 2010; 16:1969–1977.
Article28. Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006; 314:1157–1160.
Article29. Lonnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009; 12:293–297.
Article30. Legrand D, Elass E, Carpentier M, Mazurier J. Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol. 2006; 84:282–290.31. Jang YS, Seo GY, Lee JM, Seo HY, Han HJ, Kim SJ, Jin BR, Kim HJ, Park SR, Rhee KJ, Kim WS, Kim PH. Lactoferrin causes IgA and IgG2b isotype switching through betaglycan binding and activation of canonical TGF-beta signaling. Mucosal Immunol. 2014; doi: 10.1038/mi.2014.121.32. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001; 31:1706–1715.
Article33. Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000; 288:2222–2226.
Article34. Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009; 21:28–35.
Article35. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004; 21:527–538.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Control of Innate and Adaptive Lymphocytes by the RAR-Retinoic Acid Axis
- Further Characterization of Activin A-induced IgA Response in Murine B Lymphocytes
- Combined Treatment With TGF-β1, Retinoic Acid, and Lactoferrin Robustly Generate Inducible Tregs (iTregs) Against High Affinity Ligand
- Lactoferrin Stimulates Mouse Macrophage to Express BAFF via Smad3 Pathway
- Preferential Expression of IgA Isotype Switching-associated Transcripts in Mouse Intestinal Lymphoid Tissues