Hanyang Med Rev.
2011 May;31(2):123-133. 10.7599/hmr.2011.31.2.123.
Role of Sigma Receptor and Neurosteroids in Pain Sensation
- Affiliations
-
- 1Department of Veterinary Physiology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea. JHL1101@snu.ac.kr
- KMID: 2168189
- DOI: http://doi.org/10.7599/hmr.2011.31.2.123
Abstract
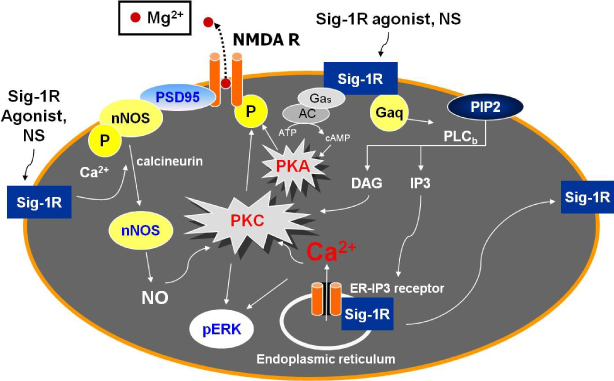
- The sigma-1 receptor has recently been implicated in a myriad of cellular functions and biological processes. Previous studies have demonstrated that the spinal sigma-1 receptor plays a pro-nociceptive role in acute pain and that the direct activation of sigma-1 receptor enhances the nociceptive response to peripheral stimuli, which is closely associated with calcium-dependent second messenger cascades including protein kinase C (PKC). In addition, the activation of sigma-1 receptor increases PKC- and protein kinase alpha (PKA)-dependent phosphorylation of the N-Methyl- D-aspartate (NMDA) receptor in the spinal cord, which results in the potentiation of intrathecal NMDA-evoked spontaneous pain behavior. Moreover, the blockade of spinal sigma-1 receptor suppresses the development of neuropathic pain and blocks the increase of phosphorylation of extracellular signal-regulated kinase (ERK) as well as pNR1 in the spinal cord. Recently, it was also reported that spinal neurosteroids such as pregnenolone and dehydroepiandrosterone sulfate, which are recognized as endogenous ligands for sigma-1 receptor, could produce mechanical hypersensitivity via sigma-1 receptor-mediated increase of pNR1. Collectively, these findings demonstrate that the activation of spinal sigma-1 receptor or the increase of neurosteroids is closely associated with the acute pain sensation or the development of chronic pain, and imply that sigma-1 receptor can be a new potential target for the development of analgesics.
Keyword
MeSH Terms
-
Acute Pain
Analgesics
Biological Processes
Central Nervous System Sensitization
Chronic Pain
D-Aspartic Acid
Dehydroepiandrosterone Sulfate
Hypersensitivity
Ligands
Neuralgia
Neurotransmitter Agents
Phosphorylation
Phosphotransferases
Pregnenolone
Protein Kinase C
Protein Kinases
Receptors, sigma
Second Messenger Systems
Sensation
Spinal Cord
Analgesics
D-Aspartic Acid
Dehydroepiandrosterone Sulfate
Ligands
Neurotransmitter Agents
Phosphotransferases
Pregnenolone
Protein Kinase C
Protein Kinases
Receptors, sigma
Figure
Reference
-
1. Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990. 42:355–402.2. Collier TL, Waterhouse RN, Kassiou M. Imaging sigma receptors: applications in drug development. Curr Pharm Des. 2007. 13:51–72.
Article3. Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996. 93:8072–8077.
Article4. Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl). 2004. 174:301–319.
Article5. Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003. 10:2073–2080.
Article6. Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002. 34:399–410.
Article7. Hayashi T, Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol. 2005. 3:267–280.
Article8. Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009. 124:195–206.
Article9. Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009. 323:934–937.
Article10. Mensah-Nyagan AG, Saredi S, Schaeffer V, Kibaly C, Meyer L, Melcangi RC, Patte-Mensah C. Assessment of neuroactive steroid formation in diabetic rat spinal cord using high-performance liquid chromatography and continuous flow scintillation detection. Neurochem Int. 2008. 52:554–559.
Article11. Kibaly C, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Biochemical and functional evidence for the control of pain mechanisms by dehydroepiandrosterone endogenously synthesized in the spinal cord. FASEB J. 2008. 22:93–104.
Article12. Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids:molecular, physiological, and behavioral aspects. J Pharmacol Sci. 2006. 100:93–118.
Article13. Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011. 300:C328–C337.14. Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000. 97:155–170.
Article15. Mensah-Nyagan AG, Kibaly C, Schaeffer V, Venard C, Meyer L, Patte-Mensah C. Endogenous steroid production in the spinal cord and potential involvement in neuropathic pain modulation. J Steroid Biochem Mol Biol. 2008. 109:286–293.
Article16. Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J Pharmacol Exp Ther. 1994. 271:1583–1590.17. Chien CC, Pasternak GW. Sigma antagonists potentiate opioid analgesia in rats. Neurosci Lett. 1995. 190:137–139.
Article18. Mei J, Pasternak GW. Sigma1 receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther. 2002. 300:1070–1074.
Article19. Marrazzo A, Parenti C, Scavo V, Ronsisvalle S, Scoto GM, Ronsisvalle G. In vivo evaluation of (+)-MR200 as a new selective sigma ligand modulating MOP, DOP and KOP supraspinal analgesia. Life Sci. 2006. 78:2449–2453.
Article20. Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Lee JH. Intrathecal administration of sigma-1 receptor agonists facilitates nociception: involvement of a protein kinase C-dependent pathway. J Neurosci Res. 2008. 86:3644–3654.
Article21. Ueda H, Inoue M, Yoshida A, Mizuno K, Yamamoto H, Maruo J, Matsuno K, Mita S. Metabotropic neurosteroid/sigma-receptor involved in stimulation of nociceptor endings of mice. J Pharmacol Exp Ther. 2001. 298:703–710.22. Cendan CM, Pujalte JM, Portillo-Salido E, Baeyens JM. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology (Berl). 2005. 182:485–493.
Article23. Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005. 511:73–74.24. Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006. 148:490–498.
Article25. Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005. 116:62–72.
Article26. Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-D-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008. 12:552–563.
Article27. Entrena JM, Cobos EJ, Nieto FR, Cendan CM, Gris G, Del Pozo E, Zamanillo D, Baeyens JM. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain. 2009. 143:252–261.
Article28. Kwon YB, Jeong YC, Kwon JK, Son JS, Kim KW. The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats. Korean J Physiol Pharmacol. 2009. 13:425–429.
Article29. Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH. Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 2008. 109:879–889.
Article30. de la Puente B, Nadal X, Portillo-Salido E, Sanchez-Arroyos R, Ovalle S, Palacios G, Muro A, Romero L, Entrena JM, Baeyens JM, Lopez-Garcia JA, Maldonado R, Zamanillo D, Vela JM. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009. 145:294–303.
Article31. Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997. 241:535–540.
Article32. Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001. 98:491–496.
Article33. Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000. 526:527–539.
Article34. Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007. 578:143–157.
Article35. Bermack JE, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005. 97:317–336.
Article36. Roh DH, Yoon SY, Seo HS, Kang SY, Moon JY, Song S, Beitz AJ, Lee JH. Sigma-1 receptor-induced increase in murine spinal NR1 phosphorylation is mediated by the PKCalpha and epsilon, but not the PKCzeta, isoforms. Neurosci Lett. 2010. 477:95–99.
Article37. Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004. 18:269–284.38. Chaki S, Okuyama S, Ogawa S, Tomisawa K. Regulation of NMDA-induced [3H]dopamine release from rat hippocampal slices through sigma-1 binding sites. Neurochem Int. 1998. 33:29–34.
Article39. Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997. 272:5157–5166.
Article40. Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008. 154:1125–1134.
Article41. Roh DH, Yoon SY, Seo HS, Kang SY, Moon JY, Song S, Beitz AJ, Lee JH. Sigma-1 receptor-induced increase in murine spinal NR1 phosphorylation is mediated by the PKCalpha and varepsilon, but not the PKCzeta, isoforms. Neurosci Lett. 2010. 477:95–99.
Article42. Zhou C, Li C, Yu HM, Zhang F, Han D, Zhang GY. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J Neurosci Res. 2008. 86:2973–2983.
Article43. Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, Kwon SG, Han HJ, Beitz AJ, Lee JH. Spinal nNOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent NR1 phosphorylation. Br J Pharmacol. 2011. Forthcoming.44. Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009. 20:223–230.
Article45. Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008. 27:2783–2802.
Article46. Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997. 21:69–102.47. Horan B, Gifford AN, Matsuno K, Mita S, Ashby CR Jr. Effect of SA4503 on the electrically evoked release of (3)H-acetylcholine from striatal and hippocampal rat brain slices. Synapse. 2002. 46:1–3.
Article48. Garrone B, Magnani M, Pinza M, Polenzani L. Effects of trazodone on neurotransmitter release from rat mossy fibre cerebellar synaptosomes. Eur J Pharmacol. 2000. 400:35–41.
Article49. Campana G, Bucolo C, Murari G, Spampinato S. Ocular hypotensive action of topical flunarizine in the rabbit: role of sigma 1 recognition sites. J Pharmacol Exp Ther. 2002. 303:1086–1094.
Article50. Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003. 64:857–864.
Article51. Yoon SY, Roh DH, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of the neurosteroid, DHEAS, produces mechanical allodynia in mice: involvement of spinal sigma-1 and GABA receptors. Br J Pharmacol. 2009. 157:666–673.
Article52. Vallee M, Mayo W, Koob GF, Le Moal M. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001. 46:273–320.53. Charalampopoulos I, Alexaki VI, Tsatsanis C, Minas V, Dermitzaki E, Lasaridis I, Vardouli L, Stournaras C, Margioris AN, Castanas E, Gravanis A. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006. 1088:139–152.
Article54. Charalampopoulos I, Alexaki VI, Lazaridis I, Dermitzaki E, Avlonitis N, Tsatsanis C, Calogeropoulou T, Margioris AN, Castanas E, Gravanis A. G protein-associated, specific membrane binding sites mediate the neuroprotective effect of dehydroepiandrosterone. FASEB J. 2006. 20:577–579.
Article55. Yoon SY, Roh DH, Seo HS, Kang SY, Moon JY, Song S, Beitz AJ, Lee JH. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology. 2010. 59:460–467.
Article56. Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell Mol Life Sci. 2010. 67:3017–3034.
Article57. Coronel MF, Labombarda F, Villar MJ, De Nicola AF, Gonzalez SL. Progesterone prevents allodynia after experimental spinal cord injury. J Pain. 2011. 12:71–83.
Article58. Fan J, Lu Y, Yu LH, Zhang Y, Ni X, Burnstock G, Ma B. Progesterone Rapidly Attenuates ATP-Evoked Transient Currents in Cultured Rat Dorsal Root Ganglion Neurons. Pharmacology. 2010. 87:36–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurosteroids and neurological disorders
- The Role of Transient Receptor Potential Channel in Pain
- Potential Role of the Sigma-1 Receptor Chaperone in the Beneficial Effects of Donepezil in Dementia with Lewy Bodies
- Benefical Effects of Sigma-1 Agonist Fluvoxamine for Tardive Dyskinesia and Tardive Akathisia in Patients with Schizophrenia: Report of Three Cases
- The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats