Immune Netw.
2012 Jun;12(3):89-95. 10.4110/in.2012.12.3.89.
Kinetic Analysis of CpG-Induced Mouse B Cell Growth and Ig Production
- Affiliations
-
- 1Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Korea.
- 2Department of Microbiology, College of Medicine, Konyang University, Daejeon 302-718, Korea. srpark@konyang.ac.kr
- 3Myunggok Medical Research Institute, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- 4Department of Biochemistry, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- KMID: 2168006
- DOI: http://doi.org/10.4110/in.2012.12.3.89
Abstract
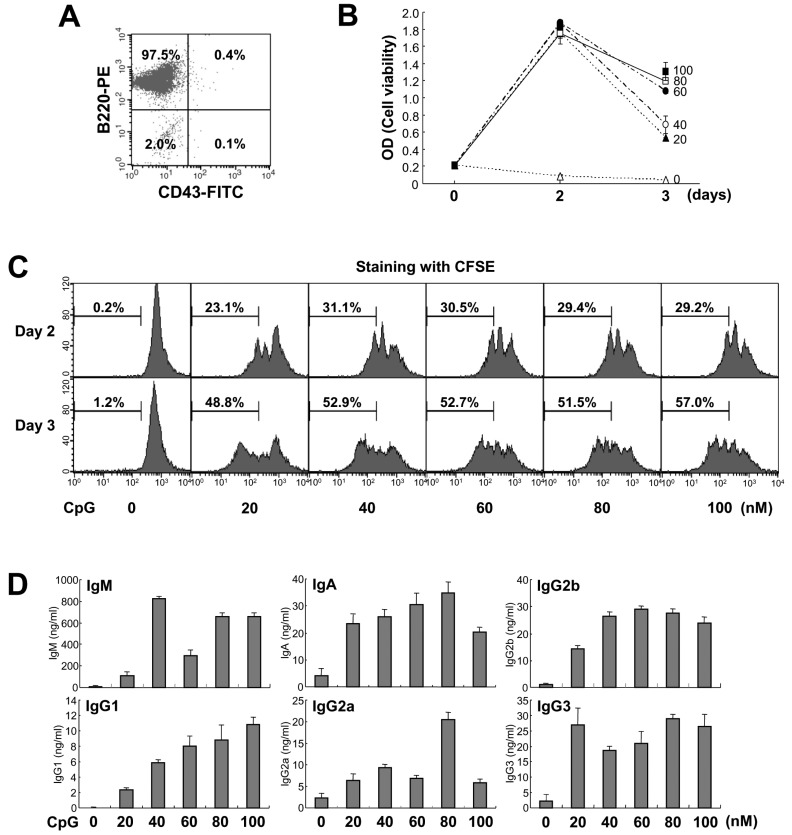
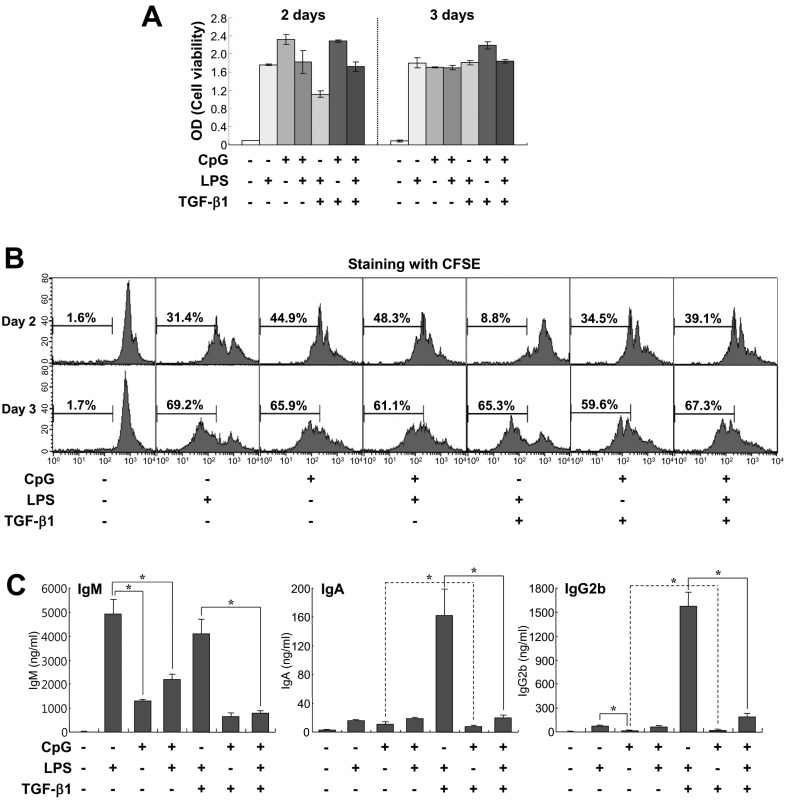
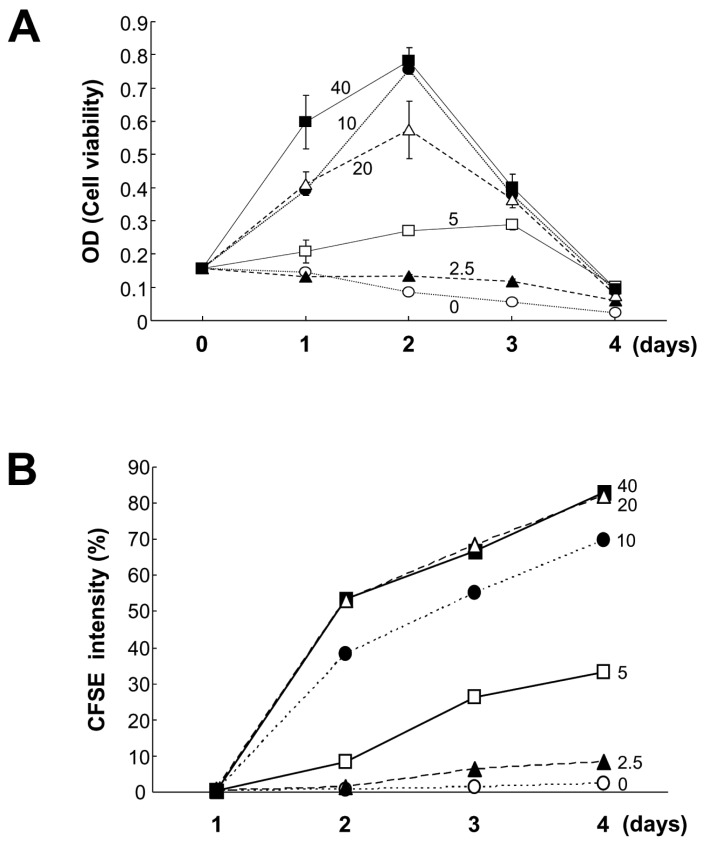
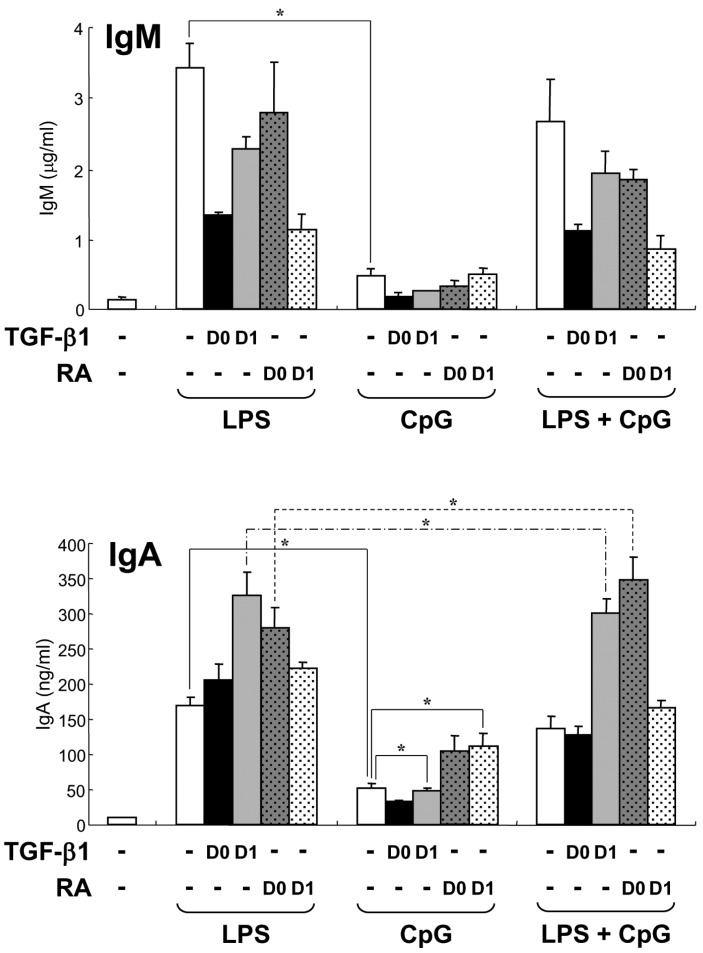
- Immune cells express toll-like receptors (TLRs) and respond to molecular patterns of various pathogens. CpG motif in bacterial DNA activates innate and acquired immune systems through binding to TLR9 of immune cells. Several studies reported that CpG can directly regulate B cell activation, differentiation, and Ig production. However, the role of CpG in B cell growth and Ig production is not fully understood. In this study, we analyzed the effect of CpG on the kinetics of mouse B cell viability, proliferation, and Igs production. Overall, CpG enhanced mouse B cell growth and production of Igs in a dose-dependent manner. Unlike LPS, 100 nM CpG (high dose) did not support TGF-beta1-induced IgA and IgG2b production. Moreover, 100 nM CpG treatment abrogated either LPS-induced IgM or LPS/TGF-beta1-induced IgA and IgG2b production, although B cell growth was enhanced by CpG under the same culture conditions. We subsequently found that 10 nM CpG (low dose) is sufficient for B cell growth. Again, 10 nM CpG did not support TGF-beta1-induced IgA production but, interestingly enough, supported RA-induced IgA production. Further, 10 nM CpG, unlike 100 nM, neither abrogated the LPS/TGF-beta1-nor the LPS/RA-induced IgA production. Taken together, these results suggest that dose of CpG is critical in B cell growth and Igs production and the optimal dose of CpG cooperates with LPS in B cell activation and differentiation toward Igs production.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Dectin-1 Stimulation Selectively Reinforces LPS-driven IgG1 Production by Mouse B Cells
Beom-Seok Seo, Sang-Hoon Lee, Ju-Eon Lee, Yung-Choon Yoo, Junglim Lee, Seok-Rae Park
Immune Netw. 2013;13(5):205-212. doi: 10.4110/in.2013.13.5.205.Ginsenoside Rg1 and 20(S)-Rg3 Induce IgA Production by Mouse B Cells
Ha-Yan Park, Sang-Hoon Lee, Kyu-Seon Lee, Hee-Kyung Yoon, Yung-Choon Yoo, Junglim Lee, Jae Eul Choi, Pyeung-Hyeun Kim, Seok-Rae Park
Immune Netw. 2015;15(6):331-336. doi: 10.4110/in.2015.15.6.331.Toll-like Receptor 1/2 Agonist Pam3CSK4 Suppresses Lipopolysaccharide-driven IgG1 Production while Enhancing IgG2a Production by B Cells
Sang-Hoon Lee, Seok-Rae Park
Immune Netw. 2018;18(1):. doi: 10.4110/in.2018.18.e10.
Reference
-
1. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801. PMID: 16497588.
Article2. Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009; 227:221–233. PMID: 19120487.
Article3. Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006; 36:810–816. PMID: 16541472.
Article4. Pone EJ, Xu Z, White CA, Zan H, Casali P. B cell TLRs and induction of immunoglobulin class-switch DNA recombination. Front Biosci. 2012; 17:2594–2615. PMID: 22652800.
Article5. Hardenberg G, Planelles L, Schwarte CM, van Bostelen L, Le Huong T, Hahne M, Medema JP. Specific TLR ligands regulate APRIL secretion by dendritic cells in a PKR-dependent manner. Eur J Immunol. 2007; 37:2900–2911. PMID: 17899538.
Article6. Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000; 290:89–92. PMID: 11021805.
Article7. Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007; 2:e863. PMID: 17848994.
Article8. Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005; 17:230–236. PMID: 15886111.
Article9. Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009; 128:311–323. PMID: 20067531.10. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995; 374:546–549. PMID: 7700380.
Article11. Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000; 164:944–953. PMID: 10623843.
Article12. Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, Rasmussen WL, Waldschmidt M, Sajuthi D, Purcell RH, Davis HL, Krieg AM. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000; 164:1617–1624. PMID: 10640783.13. Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol. 2002; 169:2368–2373. PMID: 12193703.
Article14. Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003; 4:687–693. PMID: 12766768.
Article15. Peng SL, Li J, Lin L, Gerth A. The role of T-bet in B cells. Nat Immunol. 2003; 4:1041. PMID: 14586416.
Article16. Rifkin IR, Marshak-Rothstein A. T-bet: the Toll-bridge to class-switch recombination? Nat Immunol. 2003; 4:650–652. PMID: 12830144.
Article17. Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007; 178:2415–2420. PMID: 17277148.
Article18. Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards "Th1-like" Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004; 34:1483–1487. PMID: 15114682.
Article19. Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998; 160:5898–5906. PMID: 9637502.20. Janossy G, Snajdr J, Simak-Ellis M. Patterns of B-lymphocyte gene expression elicited by lipopolysaccharide mitogen. Immunology. 1976; 30:799–810. PMID: 1088414.21. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–2088. PMID: 9851930.22. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990; 145:3773–3778. PMID: 2246513.23. Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989; 170:1039–1044. PMID: 2788703.
Article24. Lebman DA, Edmiston JS. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 1999; 1:1297–1304. PMID: 10611758.25. Tokuyama H, Tokuyama Y. Endogenous cytokine expression profiles in retinoic acid-induced IgA production by LPS-stimulated murine splenocytes. Cell Immunol. 1995; 166:247–253. PMID: 7497526.
Article26. Tokuyama Y, Tokuyama H. Retinoids as Ig isotype-switch modulators. The role of retinoids in directing isotype switching to IgA and IgG1 (IgE) in association with IL-4 and IL-5. Cell Immunol. 1996; 170:230–234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Toll-like Receptor 1/2 Agonist Pam3CSK4 Suppresses Lipopolysaccharide-driven IgG1 Production while Enhancing IgG2a Production by B Cells
- Intranasal Administration of Unmethylated CpG with Cockroach Antigen Prevents the Development of Cockroach-Induced Allergic Inflammation
- Modulation of Eosinophilia and Cytokines of Bronchoalveolar Larvage Fluid(BALF) by CpG Oligodeoxynucleotides(ODN) in a Mouse Model of Established Airway Inflammation
- Newly Identified TLR9 Stimulant, M6-395 Is a Potent Polyclonal Activator for Murine B Cells
- Role of NFkappaB in toll-like receptor 9-mediated matrix metalloproteinase-9 expression