Immune Netw.
2012 Feb;12(1):27-32. 10.4110/in.2012.12.1.27.
Newly Identified TLR9 Stimulant, M6-395 Is a Potent Polyclonal Activator for Murine B Cells
- Affiliations
-
- 1Department of Molecular Bioscience, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea. phkim@kangwon.ac.kr
- 2Department of Biological Science, College of Natural Science, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2014653
- DOI: http://doi.org/10.4110/in.2012.12.1.27
Abstract
- BACKGROUND
Toll-like receptors (TLRs) have been extensively studied in recent years. However, functions of these molecules in murine B cell biology are largely unknown. A TLR4 stimulant, LPS is well known as a powerful polyclonal activator for murine B cells.
METHODS
In this study, we explored the effect of a murine TLR9 stimulant, M6-395 (a synthetic CpG ODNs) on B cell proliferation and Ig production.
RESULTS
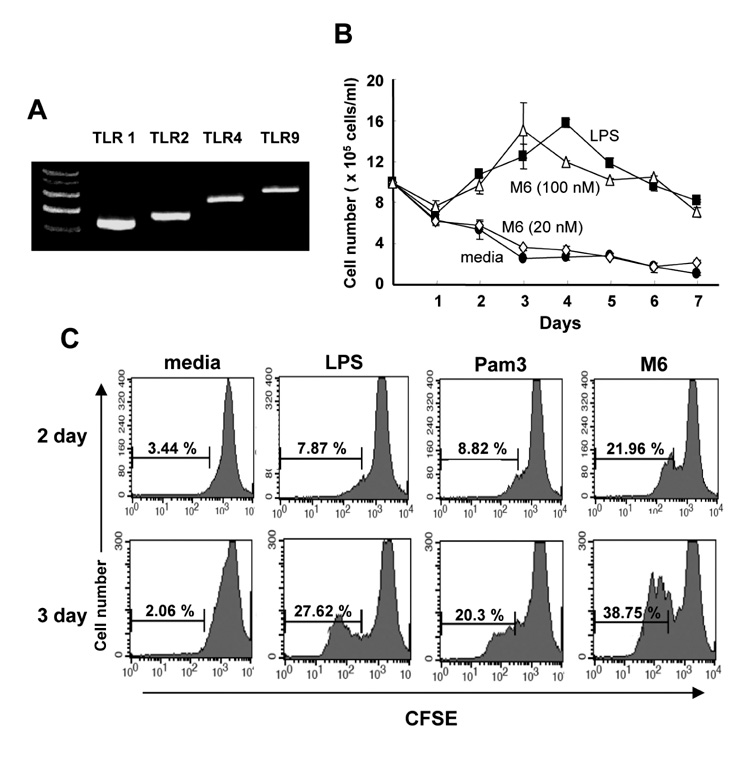
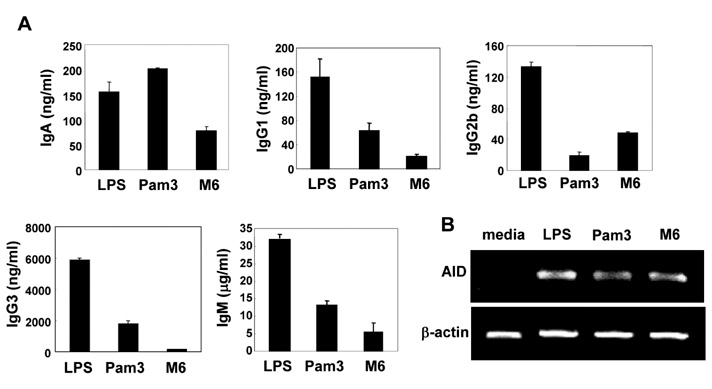
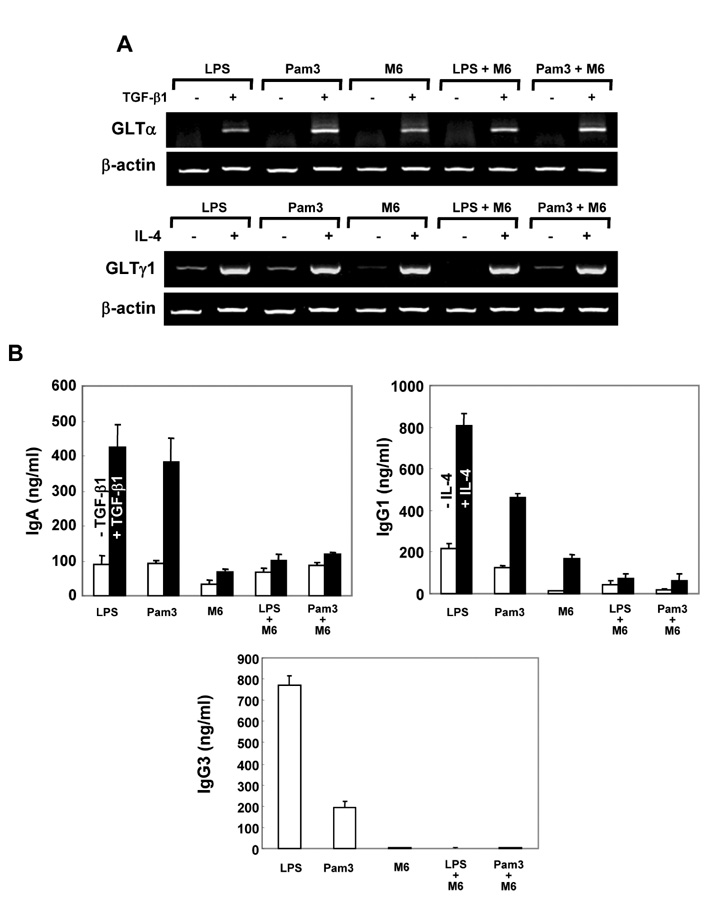
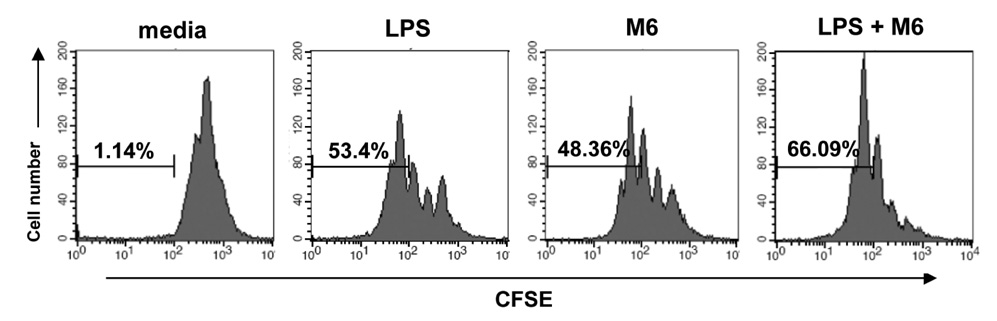
First, M6-395 was much more potent than LPS in augmenting B cell proliferation. As for Ig expression, M6-395 facilitated the expression of both TGF-beta1-induced germ line transcript alpha (GLTalpha) and IL-4-induced GLTgamma1 as levels as those by LPS and Pam3CSK4 (TLR1/2 agonist) : a certain Ig GLT expression is regarded as an indicative of the corresponding isotype switching recombination. However, IgA and IgG1 secretion patterns were quite different--these Ig isotype secretions by M6-395 were much less than those by LPS and Pam3CSK4. Moreover, the increase of IgA and IgG1 production by LPS and Pam3CSK4 was virtually abrogated by M6-395. The same was true for the secretion of IgG3. We found that this unexpected phenomena provoked by M6-395 is attributed, at least in part, to its excessive mitogenic nature.
CONCLUSION
Taken together, these results suggest that M6-395 can act as a murine polyclonal activator but its strong mitogenic activity is unfavorable to Ig isotype switching.
MeSH Terms
Figure
Reference
-
1. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007. 7:353–364.2. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004. 5:190–198.
Article3. Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006. 203:553–561.
Article4. Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007. 178:2415–2420.
Article5. Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003. 4:687–693.
Article6. Choi SS, Chung E, Jung YJ. Newly identified CpG ODNs, M5-30 and M6-395, stimulate mouse immune cells to secrete TNF-alpha and enhance Th1-mediated immunity. J Microbiol. 2010. 48:512–517.
Article7. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001. 31:1706–1715.
Article8. Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987. 139:2669–2674.9. Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J Immunol. 2005. 174:6639–6647.
Article10. Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998. 160:5898–5906.11. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000. 102:553–563.
Article12. Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000. 102:565–575.
Article13. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990. 145:3773–3778.14. Berton MT, Uhr JW, Vitetta ES. Synthesis of germ-line gamma 1 immunoglobulin heavy-chain transcripts in resting B cells: induction by interleukin 4 and inhibition by interferon gamma. Proc Natl Acad Sci U S A. 1989. 86:2829–2833.
Article15. Stavnezer J. Molecular processes that regulate class switching. Curr Top Microbiol Immunol. 2000. 245:127–168.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Limited expression of TLR9 on T cells and its functionalconsequences in patients with nonalcoholic fatty liverdisease
- Two Sjogren syndrome-associated oral bacteria, Prevotella melaninogenica and Rothia mucilaginosa, induce the upregulation of major histocompatibility complex class I and hypoxia-associated cell death, respectively, in human salivary gland cells
- Adenovirus-Mediated Antisense Vector-Induced Inhibition of Human Telomerase RNA May Induce Differentiation of CD34+ Cells
- Toll-Like Receptor Gene Expression during Trichinella spiralis Infection
- Cutaneous Plasmacytosis with Multiple Nodular Eruptions and Polyclonal Hypergammaglobulinemia