Allergy Asthma Immunol Res.
2014 Jan;6(1):6-12. 10.4168/aair.2014.6.1.6.
Histamine-Releasing Factor and Immunoglobulins in Asthma and Allergy
- Affiliations
-
- 1Division of Cell Biology, La Jolla Institute for Allergy and Immunology, La Jolla, California, USA. toshi@liai.org
- 2Laboratory of Allergic Disease, RIKEN Center for Integrative Medical Sciences (IMS-RCAI), Yokohama, Kanagawa, Japan.
- KMID: 2166922
- DOI: http://doi.org/10.4168/aair.2014.6.1.6
Abstract
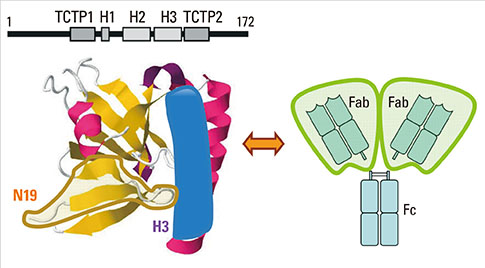
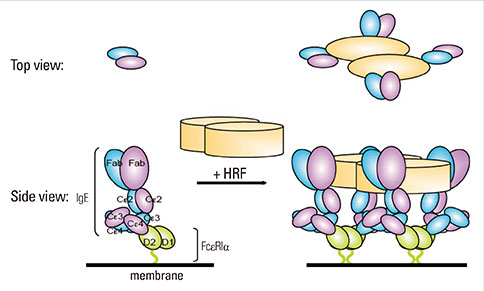
- Factors that can induce the release of histamine from basophils have been studied for more than 30 years. A protein termed histamine-releasing factor (HRF) was purified and molecularly cloned in 1995. HRF can stimulate histamine release and IL-4 and IL-13 production from IgE-sensitized basophils and mast cells. HRF-like activities were found in bodily fluids during the late phase of allergic reactions, implicating HRF in allergic diseases. However, definitive evidence for the role of HRF in allergic diseases has remained elusive. On the other hand, we found effects of monomeric IgE on the survival and activation of mast cells without the involvement of a specific antigen, as well as heterogeneity of IgEs in their ability to cause such effects. The latter property of IgE molecules seemed to be similar to the heterogeneity of IgEs in their ability to prime basophils in response to HRF. This similarity led to our recent finding that ~30% of IgE molecules can bind to HRF via their Fab interactions with two binding sites within the HRF molecule. The use of peptide inhibitors that block HRF-IgE interactions revealed an essential role of HRF to promote skin hypersensitivity and airway inflammation. This review summarizes this and more recent findings and provides a perspective on how they impact our understanding of allergy pathogenesis and potentially change the treatment of allergic diseases.
MeSH Terms
Figure
Cited by 1 articles
-
Dimerized, Not Monomeric, Translationally Controlled Tumor Protein Induces Basophil Activation and Mast Cell Degranulation in Chronic Urticaria
Bastsetseg Ulambayar, Heewon Lee, Eun-Mi Yang, Hae-Sim Park, Kyunglim Lee, Young-Min Ye
Immune Netw. 2019;19(3):. doi: 10.4110/in.2019.19.e20.
Reference
-
1. Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000; 7:32–39.2. Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999; 11:53–59.3. Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008; 8:478–486.4. Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992; 125:37–48.5. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008; 9:1215–1223.6. Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009; 228:149–169.7. Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008; 98:85–120.8. Furuichi K, Rivera J, Isersky C. The receptor for immunoglobulin E on rat basophilic leukemia cells: effect of ligand binding on receptor expression. Proc Natl Acad Sci U S A. 1985; 82:1522–1525.9. Hsu C, MacGlashan D Jr. IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett. 1996; 52:129–134.10. Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997; 185:663–672.11. Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001; 14:791–800.12. Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001; 14:801–811.13. Tanaka S, Takasu Y, Mikura S, Satoh N, Ichikawa A. Antigen-independent induction of histamine synthesis by immunoglobulin E in mouse bone marrow-derived mast cells. J Exp Med. 2002; 196:229–235.14. Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, Kawakami T. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci U S A. 2003; 100:12911–12916.15. Yamada N, Matsushima H, Tagaya Y, Shimada S, Katz SI. Generation of a large number of connective tissue type mast cells by culture of murine fetal skin cells. J Invest Dermatol. 2003; 121:1425–1432.16. Oka T, Hori M, Tanaka A, Matsuda H, Karaki H, Ozaki H. IgE alone-induced actin assembly modifies calcium signaling and degranulation in RBL-2H3 mast cells. Am J Physiol Cell Physiol. 2004; 286:C256–C263.17. Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004; 172:4048–4058.18. Lam V, Kalesnikoff J, Lee CW, Hernandez-Hansen V, Wilson BS, Oliver JM, Krystal G. IgE alone stimulates mast cell adhesion to fibronectin via pathways similar to those used by IgE + antigen but distinct from those used by Steel factor. Blood. 2003; 102:1405–1413.19. Kitaura J, Eto K, Kinoshita T, Kawakami Y, Leitges M, Lowell CA, Kawakami T. Regulation of highly cytokinergic IgE-induced mast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol. 2005; 174:4495–4504.20. Kashiwakura J, Kawakami Y, Yuki K, Zajonc DM, Hasegawa S, Tomimori Y, Caplan B, Saito H, Furue M, Oettgen HC, Okayama Y, Kawakami T. Polyclonal IgE induces mast cell survival and cytokine production. Allergol Int. 2009; 58:411–419.21. MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995; 269:688–690.22. MacDonald SM, Lichtenstein LM. Histamine-releasing factors and heterogeneity of IgE. Springer Semin Immunopathol. 1990; 12:415–428.23. Warner JA, Pienkowski MM, Plaut M, Norman PS, Lichtenstein LM. Identification of histamine releasing factor(s) in the late phase of cutaneous IgE-mediated reactions. J Immunol. 1986; 136:2583–2587.24. MacDonald SM, Lichtenstein LM, Proud D, Plaut M, Naclerio RM, MacGlashan DW, Kagey-Sobotka A. Studies of IgE-dependent histamine releasing factors: heterogeneity of IgE. J Immunol. 1987; 139:506–512.25. MacDonald SM. Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, editors. Histamine releasing factors and IgE heterogeneity. Allergy: principles and practice. 1993. 4th ed. St. Louis, MO: Mosby-Year Book Inc..26. Amzallag N, Passer BJ, Allanic D, Segura E, Théry C, Goud B, Amson R, Telerman A. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem. 2004; 279:46104–46112.27. Schroeder JT, Lichtenstein LM, MacDonald SM. An immunoglobulin E-dependent recombinant histamine-releasing factor induces interleukin-4 secretion from human basophils. J Exp Med. 1996; 183:1265–1270.28. Schroeder JT, Lichtenstein LM, MacDonald SM. Recombinant histamine-releasing factor enhances IgE-dependent IL-4 and IL-13 secretion by human basophils. J Immunol. 1997; 159:447–452.29. MacDonald SM. Human recombinant histamine-releasing factor. Int Arch Allergy Immunol. 1997; 113:187–189.30. Kleine-Tebbe J, Kagey-Sobotka A, MacGlashan DW Jr, Lichtenstein LM, MacDonald SM. Lectins do not distinguish between heterogenous IgE molecules as defined by differential activity of an IgE-dependent histamine releasing factor. J Allergy Clin Immunol. 1996; 98:181–188.31. Vonakis BM, Gibbons S Jr, Sora R, Langdon JM, MacDonald SM. Src homology 2 domain-containing inositol 5' phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J Allergy Clin Immunol. 2001; 108:822–831.32. Wantke F, MacGlashan DW, Langdon JM, MacDonald SM. The human recombinant histamine releasing factor: functional evidence that it does not bind to the IgE molecule. J Allergy Clin Immunol. 1999; 103:642–648.33. Ozeki T, Verma V, Uppalapati M, Suzuki Y, Nakamura M, Catchmark JM, Hancock WO. Surface-bound casein modulates the adsorption and activity of kinesin on SiO2 surfaces. Biophys J. 2009; 96:3305–3318.34. Kim M, Min HJ, Won HY, Park H, Lee JC, Park HW, Chung J, Hwang ES, Lee K. Dimerization of translationally controlled tumor protein is essential for its cytokine-like activity. PLoS One. 2009; 4:e6464.35. Yeh YC, Xie L, Langdon JM, Myers AC, Oh SY, Zhu Z, Macdonald SM. The effects of overexpression of histamine releasing factor (HRF) in a transgenic mouse model. PLoS One. 2010; 5:e11077.36. Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001; 19:275–290.37. Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005; 23:41–51.38. Kang HS, Lee MJ, Song H, Han SH, Kim YM, Im JY, Choi I. Molecular identification of IgE-dependent histamine-releasing factor as a B cell growth factor. J Immunol. 2001; 166:6545–6554.39. Bheekha-Escura R, MacGlashan DW, Langdon JM, MacDonald SM. Human recombinant histamine-releasing factor activates human eosinophils and the eosinophilic cell line, AML14-3D10. Blood. 2000; 96:2191–2198.40. Vonakis BM, Sora R, Langdon JM, Casolaro V, MacDonald SM. Inhibition of cytokine gene transcription by the human recombinant histamine-releasing factor in human T lymphocytes. J Immunol. 2003; 171:3742–3750.41. Yoneda K, Rokutan K, Nakamura Y, Yanagawa H, Kondo-Teshima S, Sone S. Stimulation of human bronchial epithelial cells by IgE-dependent histamine-releasing factor. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L174–L181.42. Silverman GJ, Goodyear CS. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat Rev Immunol. 2006; 6:465–475.43. Patella V, Casolaro V, Björck L, Marone G. Protein L. A bacterial Ig-binding protein that activates human basophils and mast cells. J Immunol. 1990; 145:3054–3061.44. Patella V, Florio G, Petraroli A, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000; 164:589–595.45. Patella V, Giuliano A, Florio G, Bouvet JP, Marone G. Endogenous superallergen protein Fv interacts with the VH3 region of IgE to induce cytokine secretion from human basophils. Int Arch Allergy Immunol. 1999; 118:197–199.46. Kashiwakura JC, Ando T, Matsumoto K, Kimura M, Kitaura J, Matho MH, Zajonc DM, Ozeki T, Ra C, MacDonald SM, Siraganian RP, Broide DH, Kawakami Y, Kawakami T. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J Clin Invest. 2012; 122:218–228.47. Kashiwakura J, Okayama Y, Furue M, Kabashima K, Shimada S, Ra C, Siraganian RP, Kawakami Y, Kawakami T. Most highly cytokinergic IgEs have polyreactivity to autoantigens. Allergy Asthma Immunol Res. 2012; 4:332–340.48. Valenta R, Mittermann I, Werfel T, Garn H, Renz H. Linking allergy to autoimmune disease. Trends Immunol. 2009; 30:109–116.49. Black P. Why is the prevalence of allergy and autoimmunity increasing? Trends Immunol. 2001; 22:354–355.50. Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol. 2008; 153:19–30.51. Valenta R, Seiberler S, Natter S, Mahler V, Mossabeb R, Ring J, Stingl G. Autoallergy: a pathogenetic factor in atopic dermatitis? J Allergy Clin Immunol. 2000; 105:432–437.52. Aichberger KJ, Mittermann I, Reininger R, Seiberler S, Swoboda I, Spitzauer S, Kopp T, Stingl G, Sperr WR, Valent P, Repa A, Bohle B, Kraft D, Valenta R. Hom s 4, an IgE-reactive autoantigen belonging to a new subfamily of calcium-binding proteins, can induce Th cell type 1-mediated autoreactivity. J Immunol. 2005; 175:1286–1294.53. Mittermann I, Reininger R, Zimmermann M, Gangl K, Reisinger J, Aichberger KJ, Greisenegger EK, Niederberger V, Seipelt J, Bohle B, Kopp T, Akdis CA, Spitzauer S, Valent P, Valenta R. The IgE-reactive autoantigen Hom s 2 induces damage of respiratory epithelial cells and keratinocytes via induction of IFN-gamma. J Invest Dermatol. 2008; 128:1451–1459.54. Crameri R, Faith A, Hemmann S, Jaussi R, Ismail C, Menz G, Blaser K. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. J Exp Med. 1996; 184:265–270.55. Mayer C, Appenzeller U, Seelbach H, Achatz G, Oberkofler H, Breitenbach M, Blaser K, Crameri R. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. 1999; 189:1507–1512.56. Schmid-Grendelmeier P, Flückiger S, Disch R, Trautmann A, Wüthrich B, Blaser K, Scheynius A, Crameri R. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. 2005; 115:1068–1075.57. Spitzauer S, Schweiger C, Sperr WR, Pandjaitan B, Valent P, Muhl S, Ebner C, Scheiner O, Kraft D, Rumpold H, Valenta R. Molecular characterization of dog albumin as a cross-reactive allergen. J Allergy Clin Immunol. 1994; 93:614–627.58. Valenta R, Duchêne M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, Breitenbach M, Rumpold H, Kraft D, Scheiner O. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991; 253:557–560.59. Zeller S, Rhyner C, Meyer N, Schmid-Grendelmeier P, Akdis CA, Crameri R. Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol. 2009; 124:278–285. 285.e1–285.e7.60. Feng Y, Liu D, Yao H, Wang J. Solution structure and mapping of a very weak calcium-binding site of human translationally controlled tumor protein by NMR. Arch Biochem Biophys. 2007; 467:48–57.61. Graidist P, Yazawa M, Tonganunt M, Nakatomi A, Lin CC, Chang JY, Phongdara A, Fujise K. Fortilin binds Ca2+ and blocks Ca2+-dependent apoptosis in vivo. Biochem J. 2007; 408:181–191.62. Kim M, Jung Y, Lee K, Kim C. Identification of the calcium binding sites in translationally controlled tumor protein. Arch Pharm Res. 2000; 23:633–636.63. Sanchez JC, Schaller D, Ravier F, Golaz O, Jaccoud S, Belet M, Wilkins MR, James R, Deshusses J, Hochstrasser D. Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis. 1997; 18:150–155.64. Cans C, Passer BJ, Shalak V, Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, Fiucci G, Goud B, Mirande M, Amson R, Telerman A. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci U S A. 2003; 100:13892–13897.65. Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 2006; 20:1294–1307.66. Langdon JM, Vonakis BM, MacDonald SM. Identification of the interaction between the human recombinant histamine releasing factor/translationally controlled tumor protein and elongation factor-1 delta (also known as eElongation factor-1B beta). Biochim Biophys Acta. 2004; 1688:232–236.67. Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007; 445:785–788.68. Zhang D, Li F, Weidner D, Mnjoyan ZH, Fujise K. Physical and functional interaction between myeloid cell leukemia 1 protein (MCL1) and Fortilin. The potential role of MCL1 as a fortilin chaperone. J Biol Chem. 2002; 277:37430–37438.69. Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF. Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol Cell Biol. 2005; 25:3117–3126.70. Yang Y, Yang F, Xiong Z, Yan Y, Wang X, Nishino M, Mirkovic D, Nguyen J, Wang H, Yang XF. An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene. 2005; 24:4778–4788.71. Rho SB, Lee JH, Park MS, Byun HJ, Kang S, Seo SS, Kim JY, Park SY. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011; 585:29–35.72. Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D, Colaluca I, Viale G, Rodrigues-Ferreira S, Wynendaele J, Chaloin O, Hoebeke J, Marine JC, Di Fiore PP, Telerman A. Reciprocal repression between P53 and TCTP. Nat Med. 2012; 18:91–99.73. Rinnerthaler M, Jarolim S, Heeren G, Palle E, Perju S, Klinger H, Bogengruber E, Madeo F, Braun RJ, Breitenbach-Koller L, Breitenbach M, Laun P. MMI1 (YKL056c, TMA19), the yeast orthologue of the translationally controlled tumor protein (TCTP) has apoptotic functions and interacts with both microtubules and mitochondria. Biochim Biophys Acta. 2006; 1757:631–638.74. Bommer UA. Cellular function and regulation of the translationally controlled tumour protein TCTP. Open Allergy J. 2012; 5:19–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histamine releasibilityfrom peripheral basophils in exercise-induced asthma
- The effect of histamine on the production of interferongamma and interleukin-12 in peripheral blood mononuclear cells from patients with atopic dermatitis
- Use of Intravenous Immunoglobulin in the Treatment of Childhood Atopic Dermatitis
- Spontaneous Histamine Release and Brochial Hyperreactivity in Allergic Asthma
- Basophil histamine releasability in children with atopic asthma