Cancer Res Treat.
2004 Jun;36(3):199-204.
The Efficacy of a Modified Chronomodulated Infusion of Oxaliplatin, 5-Fluorouracil and Leucovorin in Advanced Colorectal Cancer (Preliminary Data)
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea. sykim55@chollian.net
Abstract
- PURPOSE
To determine the efficacy and tolerability of a modified chronomodulated infusion of oxaliplatin, 5-fluorouracil (5-FU) and leucovorin in the treatment of advanced colorectal cancer.
MATERIALS AND METHODS
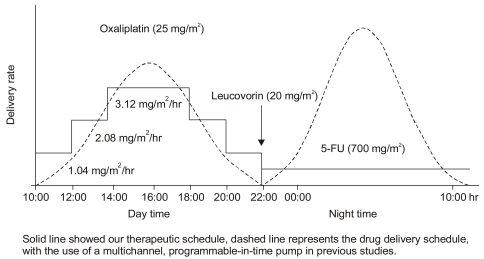
Sixteen patients with relapsed or metastatic colorectal cancer were treated with an intravenous infusion of oxaliplatin 25 mg/m(2), 5-FU 700 mg/m(2) and leucovorin 20 mg/m(2) on days 1 to 5. The infusion of oxaliplatin was chronomodulated with a peak delivery rate at 16: 00 p.m., with 5-FU infused constantly overnight. Each course was repeated every 21 days.
RESULTS
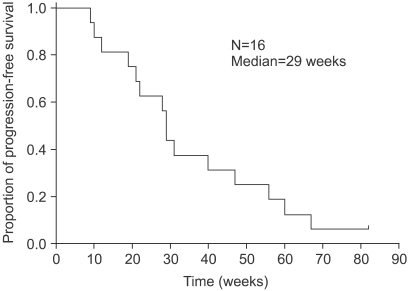
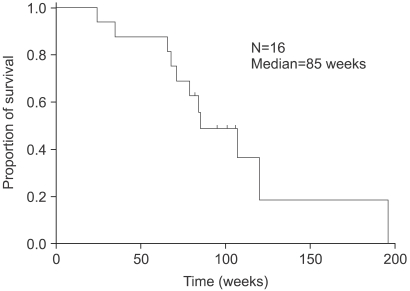
The response rate was 38.5% (95% confidence interval [CI], 13.9% to 68.4%) in the 13 measurable patients, including 1 complete response (7.7%) and 4 partial responses (30.8%). Five patients (38.5%) had a stable disease and 3 (23.0%) a progressive disease. Three patients without a measurable lesion had improved status. The median time to progression and overall survival were 29 weeks and 85 weeks, respectively. Grade 3 thrombocytopenia occurred in 2.5% (2 cycles) and grade 3 vomiting in 12.5% (2 patients). Anorexia, stomatitis, diarrhea, pruritus, alopecia and peripheral neuropathy were mild and tolerable.
CONCLUSION
The modified chronomodulated infusion of oxaliplatin, 5-FU and leucovorin is effective and tolerable, but the number of patients was too small. Further study will be needed to confirm the efficacy of this regimen with a larger population of patients.
MeSH Terms
Figure
Reference
-
1. Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998; 352:1407–1412. PMID: 9807986.
Article2. Levi F, Perpoint B, Garufi C, Focan C, Chollet P, Depres-Brummer P, Zidani R, Brienza S, Itzhaki M, Iacobelli S, Kunstlinger F, Gastiaburu J, Misset JL. Oxaliplatin activity against metastatic colorectal cancer: A phase II study of 5-day continuous venous infusion at circadian rhythm modulated rate. Eur J Cancer. 1993; 29:1280–1284. PMID: 8343268.
Article3. Levi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declere A, Jasmin C, Bismuth H, Reinberg A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump: High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992; 69:893–900. PMID: 1735081.
Article4. Levi F, Zidani R, Brienza S, Dogliotti L, Perpoint B, Rotarski M, Letourneau Y, Llory JF, Chollet P, Rol AL, Focan C. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and Leucovorin as initial treatment of patients with metastatic colorectal carcinoma. Cancer. 1999; 85:2532–2540. PMID: 10375099.
Article5. Levi F, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L, Iacobelli S, Adam R, Kunstlinger F, Gastiaburu J, Bismuth H, Jasmin C, Misset JL. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid in patients with colorectal cancer metastases. A randomized multi-institutional trial. J Natl Cancer Inst. 1994; 86:1608–1617. PMID: 7932825.6. Levi F, Zidani R, Misset JL. Randomised multicentred trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. For the International Organization for Cancer Chronotherapy. Lancet. 1997; 350:681–686. PMID: 9291901.7. Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997; 89:655–667. PMID: 9160756.
Article8. Boughattas NA, Levi F, Fournier C, Lemaigre G, Roulon A, Hecquet B, Mathe G, Reinberg A. Circadian rhythm in toxicities and tissue uptake of 1,2-diamminocyclohexane (trans-1) oxalatoplatinum (II) in mice. Cancer Res. 1989; 49:3362–3368. PMID: 2720689.9. Harris BE, Song R, Soong S, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990; 50:197–201. PMID: 2293556.10. Zhang R, Lu Z, Liu T, Soong SJ, Diasio RB. Relationship between circadian-dependent toxicity of 5-fluorodeoxyuridine and circadian rhythms of pyrimidine enzymes: possible relevance to fluoropyrimidine chemotherapy. Cancer Res. 1993; 53:2816–2822. PMID: 8504424.11. Giacchetti S, Perpoint B, Zidani R, Bail NL, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Rol AL, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000; 18:136–147. PMID: 10623704.
Article12. Advanced Colorectal Cancer Meta-Analysis Project: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: Evidence in terms of response rate. J Clin Oncol. 1992; 10:896–903. PMID: 1534121.13. Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. Prospective randomized comparison of continuous infusion of fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program study. J Clin Oncol. 1989; 7:425–432. PMID: 2926468.14. Meta-Analysis Group in Cancer: Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998; 16:301–308. PMID: 9440757.15. Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993; 53:5970–5976. PMID: 8261411.16. Tashiro T, Kawada Y, Sakurai Y, Kidani Y. Antitumor activity of a new platinum complex, oxalato (trans-1-1,2-diaminocyclohexane) platinum (II): New experimental data. Biomed Pharmacother. 1989; 43:251–260. PMID: 2790145.17. Mathe G, Kidani Y, Segiguchi M, Eriguchi M, Fredj G, Peytavin G, Misset JL, Brienza S, de Vassals F, Chenu E, Bourutet C. Oxalato-platinum or I-OHP, a third generation platinum complex: An experimental and clinical appraisal and preliminary comparison with cisplatinum and carboplatinum. Biomed Pharmacother. 1989; 43:237–250. PMID: 2675999.18. Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, Itzhaki M, Metzger G, N'Daw D, Vignoud J, Abad A, Francois E, Gamelin E, Marty M, Sastre J, Seitz JF, Ychou M. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996; 7:95–98. PMID: 9081400.
Article19. Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M. Oxaliplatin as a single agent in previously untreated colorectal carcinoma patients: A phase II multicentric study. Ann Oncol. 1998; 9:105–108. PMID: 9541691.20. Becouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL, Duffour J, Fandi A, Dupont-Andre G, Rougier P. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol. 1998; 16:2739–2744. PMID: 9704726.21. Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, Chen WS, Jiang JK, Yang SH, Wang HS, Chen PM. Tumor marker CEA in monitoring of response to tegafur-uracil and folinic acid in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002; 49:388–392. PMID: 11995458.22. Bae YZ, Jung JH, Moon CH, Kim SH, Kwon HC, Kim JS, Kim HJ. A phase II study of oxaliplatin combined with 5-fluorouracil and leucovorin (Mayo Clinic Regimen) in 5-fluorouracil refractory colorectal cancer. Cancer Res Treat. 2002; 34:218–222.
Article23. Maindrault-Goebel F, Louvet C, Andre T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, de Gramont A. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). Eur J Cancer. 1999; 35:1338–1342. PMID: 10658524.
Article24. Andre T, Louvet C, Raymond E, Tournigand C, de Gramont A. Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol. 1998; 9:1251–1253. PMID: 9862058.25. Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999; 17:3560–3568. PMID: 10550155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison between Responder and Non- responder of Oxaliplatin Chemotherapy for Metastatic Colorectal Cancer
- Combination Chemotherapy of Oxaliplatin, 5-Fluorouracil and Low Dose Leucovorin in Patients with Advanced Colorectal Cancer
- 5-fluorouracil and low dose leucovorin in advanced colorectal carcinoma
- Diffuse alveolar damage during chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin
- 5-fluorouracil and low dose leucovorin in advanced colorectal carcinoma