Cancer Res Treat.
2010 Dec;42(4):225-234.
The CXCR4 Antagonist AMD3100 Has Dual Effects on Survival and Proliferation of Myeloma Cells In Vitro
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea. deogyeon@cnu.ac.kr
- 2Daejeon Regional Cancer Center, Daejeon, Korea.
Abstract
- PURPOSE
AMD3100, an antagonist of the CXCR4 chemokine receptor is soon to be used clinically for the peripheral mobilization of hematopoietic stem cells (HSCs) in patients with multiple myeloma. AMD3100 has been shown to activate a G protein coupled with CXCR4 and thus acts as a partial CXCR4 agonist in vitro. Thus, we explored whether AMD3100 affected the survival and proliferation of myeloma cells in vitro.
MATERIALS AND METHODS
The effects of AMD3100 on survival and proliferation of two myeloma cell lines (RPMI8226 and U266) as well as CD138+ cells obtained from several patients with multiple myeloma were analyzed by flow cytometry using annexin V and a colorimetric cell proliferation assay (CCK-8 assay).
RESULTS
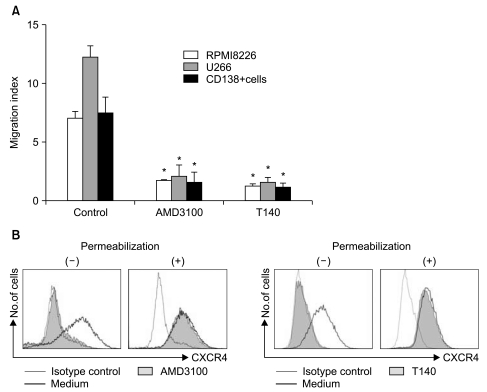
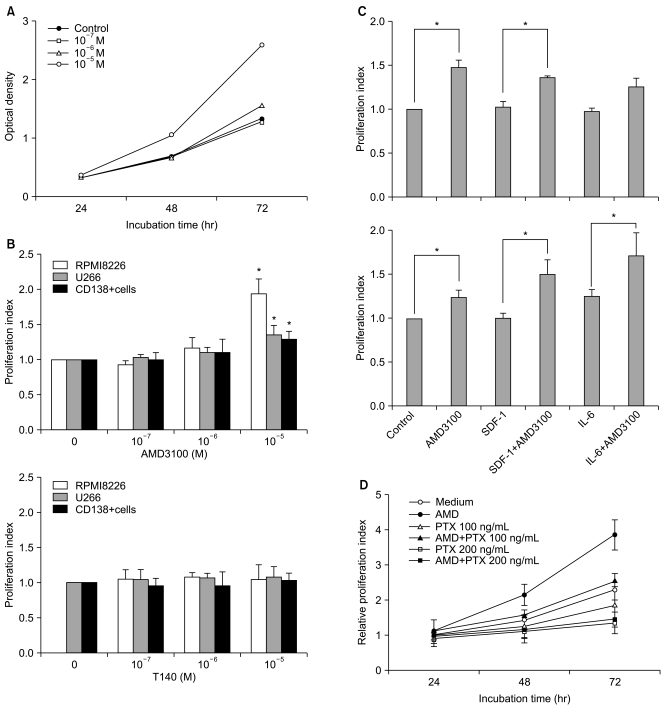
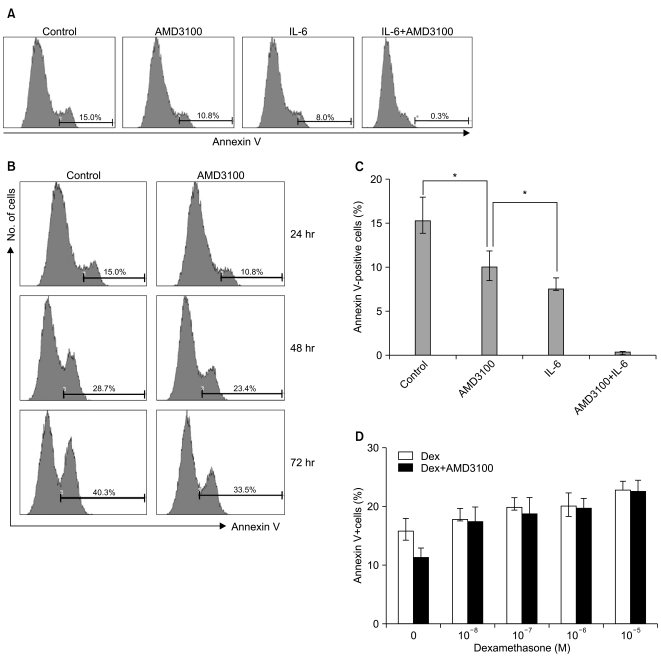
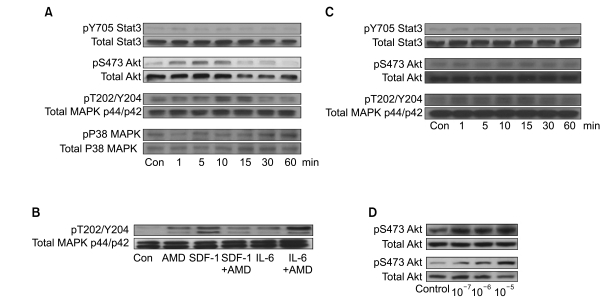
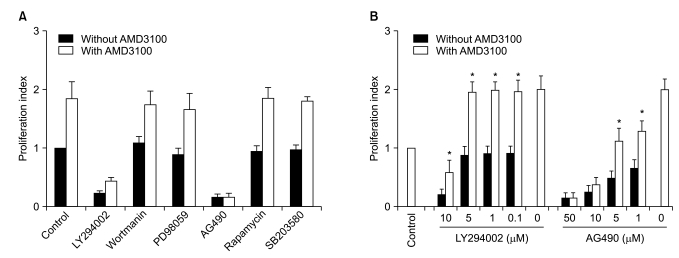
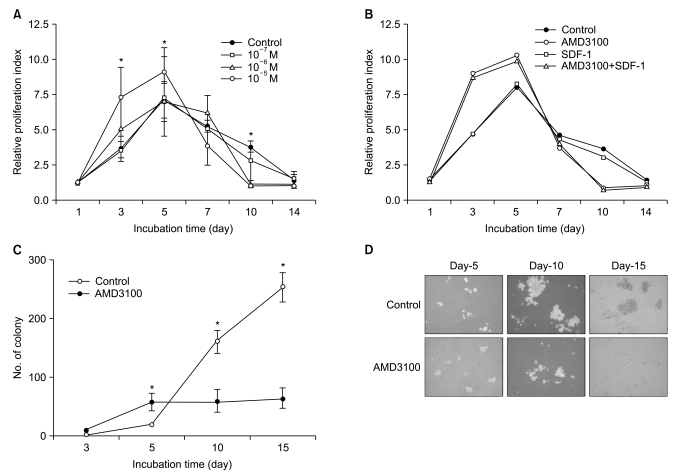
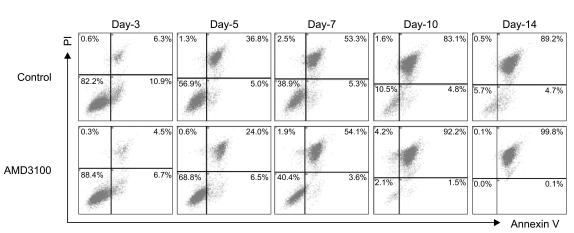
AMD3100, but not T140, another CXCR4 antagonist, stimulated the proliferation of myeloma cell lines and CD138+ primary human myeloma cells (-2-fold increase) in a dose-dependent manner in serum-free culture for up to 5 days, which was inhibited by pretreating the cells with pertussis toxin. AMD3100 enhanced the proliferation of U266 cells induced by interleukin-6 and partially reversed AG490-mediated growth inhibition and apoptosis induced by serum deprivation in RPMI8226 cells. AMD3100 induced the phosphorylation of Akt and MAPK p44/p42 in U266 cells and MAPK p44/p42 in RPMI8226 cells. In contrast, AMD3100 markedly increased the cell apoptosis and reduced the number of RPMI8226 cells after 5 to 7 days of culture under serum-free conditions.
CONCLUSION
AMD3100 exerts dual effects, initially enhancing and subsequently inhibiting the survival and proliferation of myeloma cells, signaling via CXCR4 in vitro.
Keyword
MeSH Terms
Figure
Reference
-
1. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848. PMID: 9933168.
Article2. Dürig J, Schmücker U, Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001; 15:752–756. PMID: 11368435.
Article3. Sanz-Rodríguez F, Hidalgo A, Teixidó J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001; 97:346–351. PMID: 11154207.4. Hideshima T, Chauhan D, Hayashi T, Podar K, Akiyama M, Gupta D, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002; 1:539–544. PMID: 12479272.5. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005; 105:3793–3801. PMID: 15677562.
Article6. Vande Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, et al. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica. 2006; 91:200–206. PMID: 16461304.7. Pellegrino A, Ria R, Di Pietro G, Cirulli T, Surico G, Pennisi A, et al. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br J Haematol. 2005; 129:248–256. PMID: 15813853.
Article8. Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005; 65:5864–5871. PMID: 15994964.
Article9. Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006; 107:3772–3778. PMID: 16439684.
Article10. Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005; 45:295–300. PMID: 15752146.
Article11. Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009; 113:6206–6214. PMID: 19050309.
Article12. Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009; 113:4341–4351. PMID: 19139079.
Article13. Grignani G, Perissinotto E, Cavalloni G, Carnevale Schianca F, Aglietta M. Clinical use of AMD3100 to mobilize CD34+ cells in patients affected by non-Hodgkin's lymphoma or multiple myeloma. J Clin Oncol. 2005; 23:3871–3872. PMID: 15923599.
Article14. Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 2002; 277:24515–24521. PMID: 11923301.
Article15. Watanabe M, Matsuyama W, Shirahama Y, Mitsuyama H, Oonakahara K, Noma S, et al. Dual effect of AMD3100, a CXCR4 antagonist, on bleomycin-induced lung inflammation. J Immunol. 2007; 178:5888–5898. PMID: 17442973.
Article16. Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004; 35:233–245. PMID: 15339043.
Article17. Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008; 22:2151–5158. PMID: 18769446.
Article18. Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001; 167:4686–4692. PMID: 11591799.19. Liesveld JL, Bechelli J, Rosell K, Lu C, Bridger G, Phillips G 2nd, et al. Effects of AMD3100 on transmigration and survival of acute myelogenous leukemia cells. Leuk Res. 2007; 31:1553–1563. PMID: 17403536.
Article20. Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro. World J Gastroenterol. 2008; 14:2308–2313. PMID: 18416455.21. Diamond P, Labrinidis A, Martin SK, Farrugia AN, Gronthos S, To LB, et al. Targeted disruption of the CXCL12/CXCR4 axis inhibits osteolysis in a murine model of myeloma-associated bone loss. J Bone Miner Res. 2009; 24:1150–1161. PMID: 19335218.
Article22. Trent JO, Wang ZX, Murray JL, Shao W, Tamamura H, Fujii N, et al. Lipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonists. J Biol Chem. 2003; 278:47136–47144. PMID: 12958314.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential effects of CXCR4 antagonists on the survival and proliferation of myeloid leukemia cells in vitro
- Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis
- Bortezomib inhibits the survival and proliferation of bone marrow stromal cells
- Bone marrow stem/progenitor cell mobilization in C57BL/6J and BALB/c mice
- Extracellular Hsp70 Is Involved in the CXCL12/CXCR4 Pathway in Primary Human Nasal Epithelial Cells: A Preliminary Study