Blood Res.
2015 Jun;50(2):87-96. 10.5045/br.2015.50.2.87.
Bortezomib inhibits the survival and proliferation of bone marrow stromal cells
- Affiliations
-
- 1Department of Drug Activity, New Drug Development Center, Medical Innovation Foundation, Osong, Korea.
- 2Department of Internal Medicine, School of Medicine, Chungnam National University, Daejeon, Korea. deogyeon@cnu.ac.kr
- KMID: 2172772
- DOI: http://doi.org/10.5045/br.2015.50.2.87
Abstract
- BACKGROUND
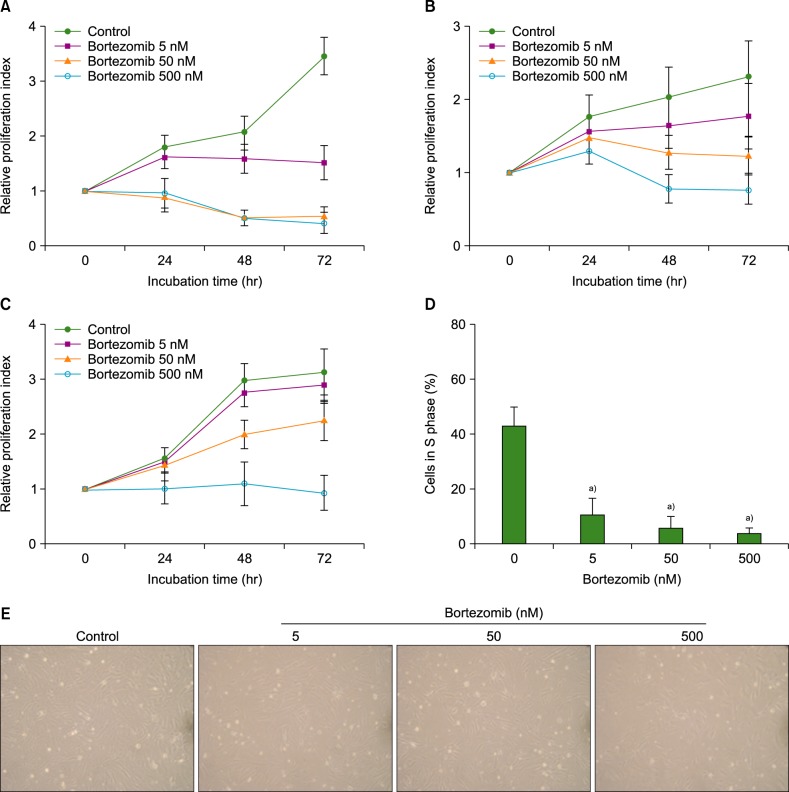
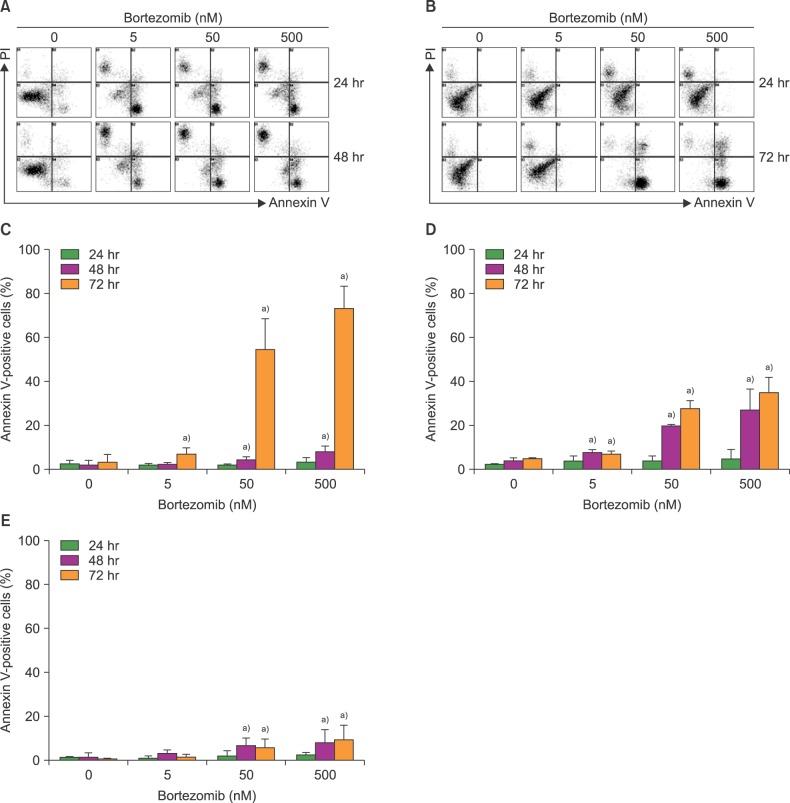
Bortezomib is widely used for the treatment of multiple myeloma. Bone marrow stromal cells (BMSCs) endow myeloma cells with survival and growth advantages. However, the influence of bortezomib on BMSCs is not well elucidated. We examined the effects of bortezomib on the survival and growth of BMSCs in vitro.
METHODS
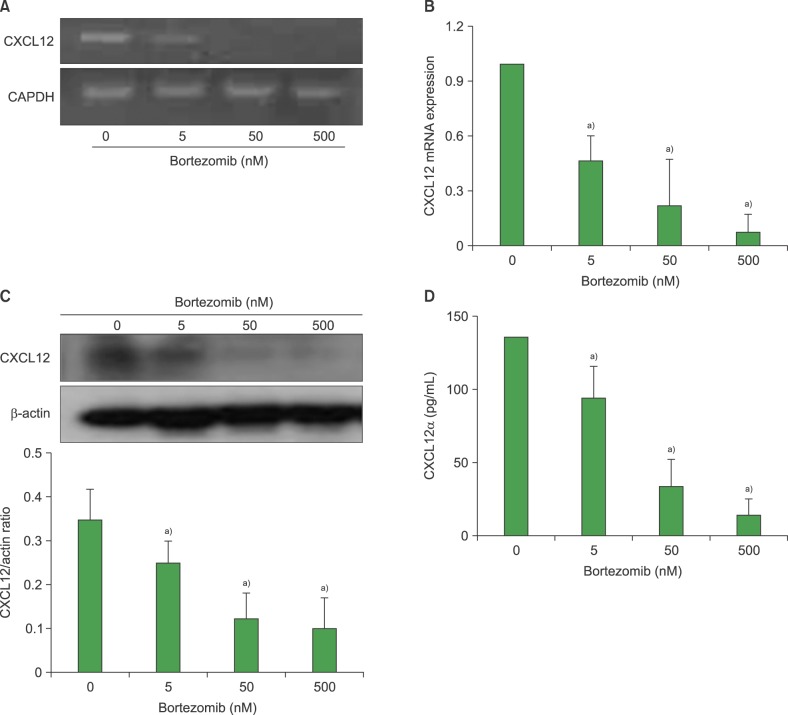
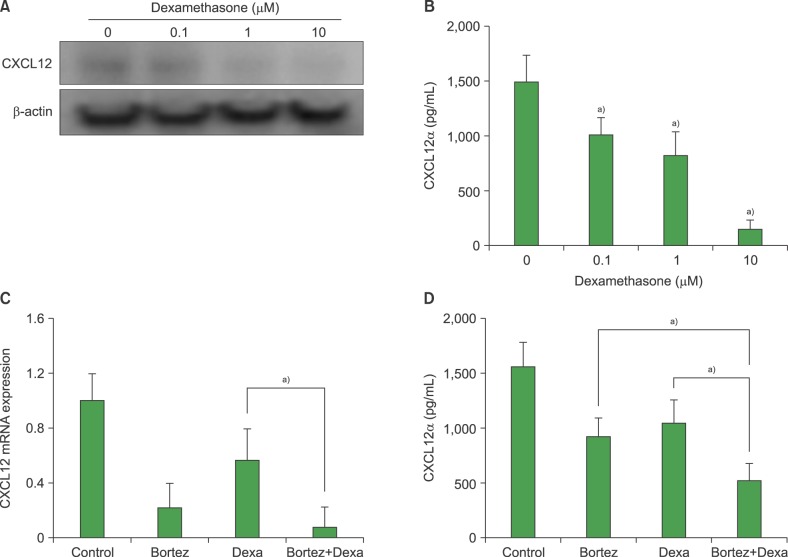
The effects of bortezomib on the survival and proliferation of the BMSC MS-5 cell line and on BMSCs obtained from healthy individuals (N=4) and newly diagnosed myeloma patients (N=5) were investigated in vitro. Transmembrane cell migration was evaluated using the Transwell system. A short interfering RNA strategy was used to knock down the expression of chemokine (CXC motif) ligand 12 (CXCL12) mRNA. To examine the effects of bortezomib-exposed BMSCs on the migration and localization of myeloma cells, MS-5 monolayers were treated with bortezomib for 24 hr, washed, and then overlaid with human RPMI8226 myeloma cells.
RESULTS
Bortezomib inhibited BMSC proliferation in a concentration-dependent manner, and induced cellular apoptosis. Bortezomib decreased CXCL12 production by BMSCs. Knockdown of CXCL12 mRNA in BMSCs revealed that CXCL12 served as an autocrine growth factor. Short-term bortezomib treatment of BMSC monolayers reduced the tendency of myeloma cells to locate to positions under the monolayers.
CONCLUSION
Bortezomib inhibits the survival and growth of BMSCs via downregulation of CXCL12, which may contribute to the clinical effects of this agent.
MeSH Terms
Figure
Reference
-
1. Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001; 61:3071–3076. PMID: 11306489.2. Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003; 101:2377–2380. PMID: 12424198.
Article3. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348:2609–2617. PMID: 12826635.
Article4. Huang N, Kawano MM, Harada H, et al. Heterogeneous expression of a novel MPC-1 antigen on myeloma cells: possible involvement of MPC-1 antigen in the adhesion of mature myeloma cells to bone marrow stromal cells. Blood. 1993; 82:3721–3729. PMID: 8260709.
Article5. Okado T, Hawley RG. Adhesion molecules involved in the binding of murine myeloma cells to bone marrow stromal elements. Int J Cancer. 1995; 63:823–830. PMID: 8847141.6. Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993; 82:3712–3720. PMID: 8260708.
Article7. Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996; 87:1104–1112. PMID: 8562936.
Article8. Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999; 93:1658–1667. PMID: 10029595.
Article9. Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene. 2003; 22:2417–2421. PMID: 12717418.10. Wang X, Li C, Ju S, Wang Y, Wang H, Zhong R. Myeloma cell adhesion to bone marrow stromal cells confers drug resistance by microRNA-21 up-regulation. Leuk Lymphoma. 2011; 52:1991–1998. PMID: 21718132.
Article11. Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001; 91:1219–1230. PMID: 11283920.
Article12. Feng Y, Wen J, Mike P, et al. Bone marrow stromal cells from myeloma patients support the growth of myeloma stem cells. Stem Cells Dev. 2010; 19:1289–1296. PMID: 20121456.
Article13. Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002; 99:4525–4530. PMID: 12036884.
Article14. Lazarini M, Traina F, Winnischofer SM, Costa FF, Queiroz ML, Saad ST. Effects of thalidomide on long-term bone marrow cultures from patients with myelodysplastic syndromes: induction of IL-10 expression in the stromal layers. Leuk Res. 2011; 35:1102–1107. PMID: 21511336.
Article15. Wobus M, Benath G, Ferrer RA, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol. 2012; 40:867–876. PMID: 22705469.
Article16. Roccaro AM, Hideshima T, Raje N, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006; 66:184–191. PMID: 16397231.
Article17. Maruyama D, Watanabe T, Heike Y, et al. Stromal cells in bone marrow play important roles in pro-inflammatory cytokine secretion causing fever following bortezomib administration in patients with multiple myeloma. Int J Hematol. 2008; 88:396–402. PMID: 18989635.
Article18. Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000; 105:101–111. PMID: 10619866.
Article19. Barbieri F, Bajetto A, Stumm R, et al. Overexpression of stromal cell-derived factor 1 and its receptor CXCR4 induces autocrine/paracrine cell proliferation in human pituitary adenomas. Clin Cancer Res. 2008; 14:5022–5032. PMID: 18698020.
Article20. Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010; 107:20009–20014. PMID: 21041659.21. Lee HJ, Lee K, Lee DG, et al. Chemokine (C-X-C motif) ligand 12 is associated with gallbladder carcinoma progression and is a novel independent poor prognostic factor. Clin Cancer Res. 2012; 18:3270–3280. PMID: 22553346.
Article22. Kim SW, Hwang JH, Jin SA, et al. Role of pertussis toxin-sensitive G protein-coupled receptor signaling in the proliferation of bone marrow mesenchymal stem cells. Korean J Hematol. 2007; 42:24–32.
Article23. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005; 105:3793–3801. PMID: 15677562.
Article24. Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000; 106:1331–1339. PMID: 11104786.
Article25. Psenák O, Sefc L, Sýkora V, Chang KT, Necas E. Cytokine gene expression in regenerating haematopoietic tissues of mice after cyclophosphamide treatment. Acta Haematol. 2003; 109:68–75. PMID: 12624490.
Article26. Lerman OZ, Greives MR, Singh SP, et al. Low-dose radiation augments vasculogenesis signaling through HIF-1-dependent and -independent SDF-1 induction. Blood. 2010; 116:3669–3676. PMID: 20631377.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Effects of dexamethasone on proliferation, collagen synthesis and alkaline phosphatase activity of normal human bone marrow stromal cells
- Generation and Characterization of 1H8 monoclonal antibody against human bone marrow stromal cells
- An Immune-compromised Method for Tooth Transplantation Using Adult Bone Marrow Stromal Cells and Embryonic Tooth Germ
- Comparison of Bone Marrow Stromal Cells with Fibroblasts in Wound Healing Accelerating Growth Factor Secretion