Lab Anim Res.
2014 Mar;30(1):14-20. 10.5625/lar.2014.30.1.14.
Bone marrow stem/progenitor cell mobilization in C57BL/6J and BALB/c mice
- Affiliations
-
- 1Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea. kspark@snu.ac.kr
- 2Innovative Research Institute for Cell Therapy, Seoul National University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2312115
- DOI: http://doi.org/10.5625/lar.2014.30.1.14
Abstract
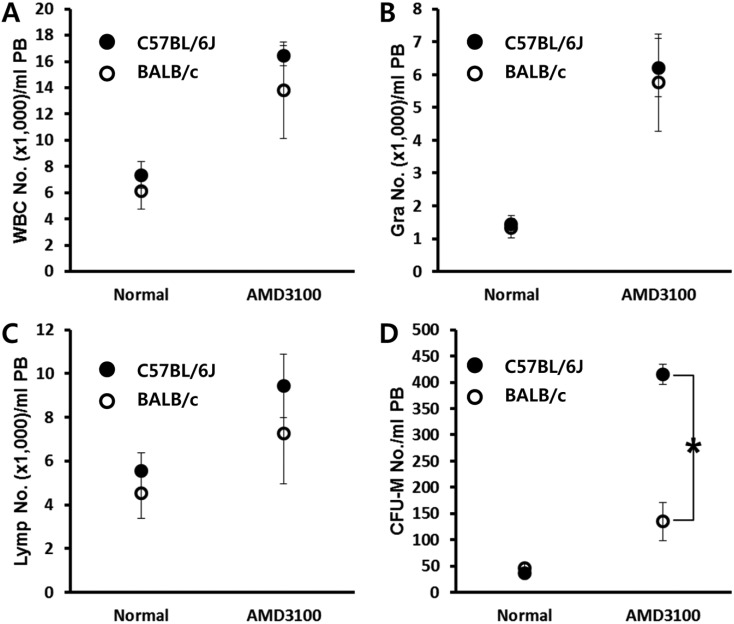
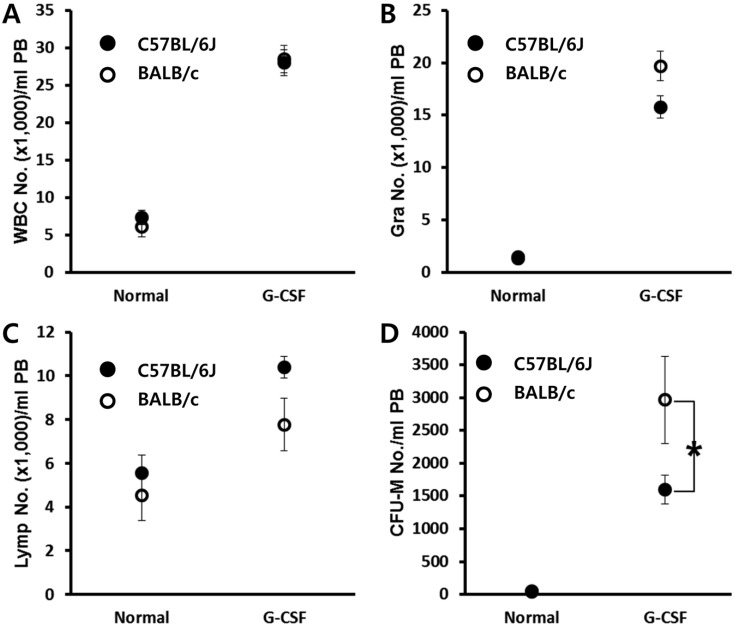
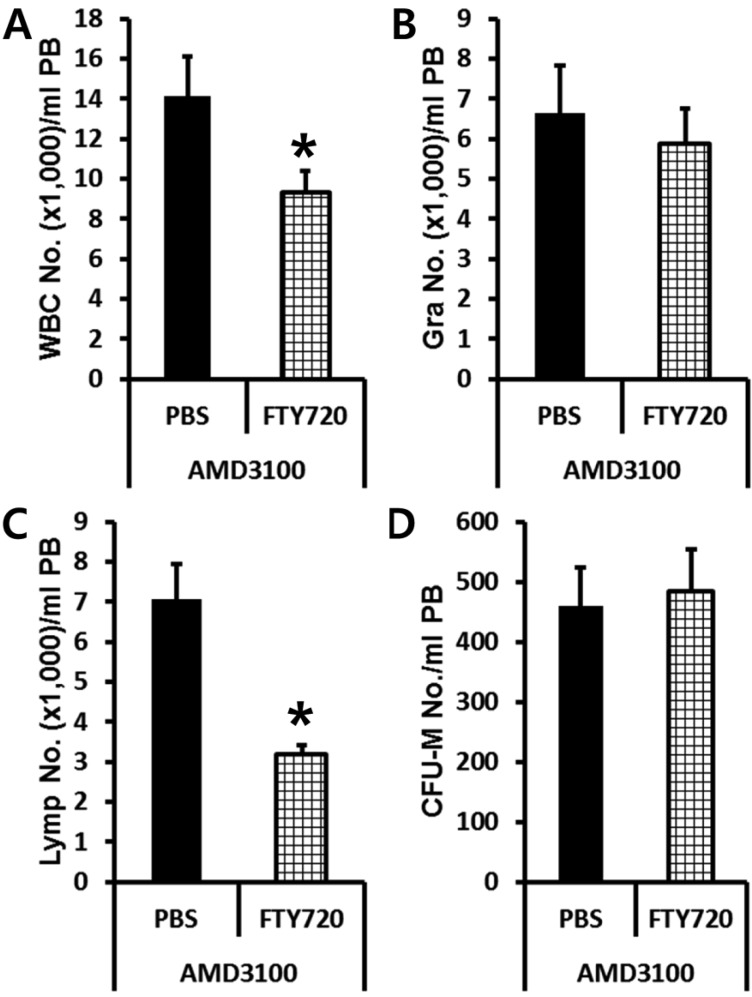
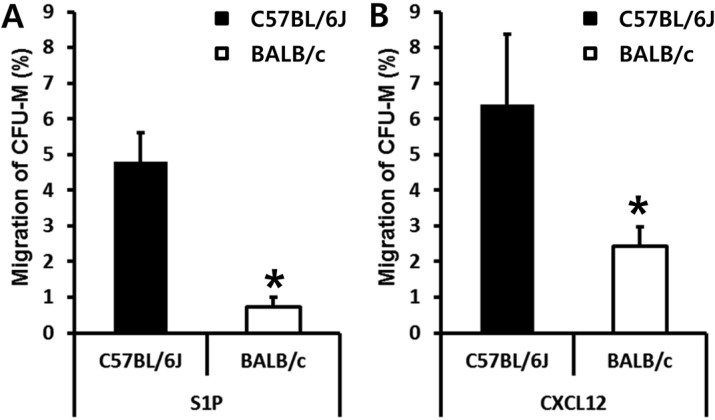
- Bone marrow (BM) has been considered as a reservoir of stem/progenitor cells which are able to differentiate into ectodermal, endodermal, and mesodermal origins in vitro as well as in vivo. Following adequate stimulation, such as granulocyte stimulating factor (G-CSF) or AMD3100, BM resident stem/progenitor cells (BMSPCs) can be mobilized to peripheral blood. Several host-related factors are known to participate in this mobilization process. In fact, a significant number of donors are resistant to G-CSF induced mobilization protocols. AMD3100 is currently used in combination with G-CSF. However, information regarding host-related factors which may influence the AMD3100 directed mobilization is extremely limited. In this study, we were to get some more knowledge on the host-related factors that affect the efficiency of AMD3100 induced mobilization by employing in vivo mobilization experiments. As a result, we found that C57BL/6J mice are more sensitive to AMD3100 but less sensitive to G-CSF which promotes the proliferation of BMSPCs. We excluded S1P as one of the host related factor which influences AMD3100 directed mobilization because pre-treatment of S1P receptor antagonist FTY720 did not inhibit BMSPC mobilization. Further in vitro experiments revealed that BALB/c mice, compared to C57BL/6J mice, have less BMSPCs which migrate in response to host related factors such as sphingosine-1-phosphate (S1P) and to CXCL12. We conclude that AMD3100-directed mobilization depends on the number of BMSPCs rather than on the host-related factors. These results suggest that the combination of AMD3100 and G-CSF is co-operative and is optimal for the mobilization of BMSPCs.
MeSH Terms
Figure
Reference
-
1. Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003; 102(4):1249–1253. PMID: 12714498.
Article2. Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000; 105(1):71–77. PMID: 10619863.
Article3. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003; 121(2):368–374. PMID: 12694261.4. Iskovich S, Goldenberg-Cohen N, Stein J, Yaniv I, Fabian I, Askenasy N. Elutriated stem cells derived from the adult bone marrow differentiate into insulin-producing cells in vivo and reverse chemical diabetes. Stem Cells Dev. 2012; 21(1):86–96. PMID: 21457125.
Article5. Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001; 98(10):2900–2908. PMID: 11698269.6. Lee H, Che JH, Lee JC, Chung SS, Jung HS, Park KS. Granulocyte-derived cationic Peptide enhances homing and engraftment of bone marrow stem cells after transplantation. Lab Anim Res. 2011; 27(2):133–140. PMID: 21826173.
Article7. Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000; 18(6):1360–1377. PMID: 10715309.
Article8. Papayannopoulou T, Nakamoto B, Andrews RG, Lyman SD, Lee MY. In vivo effects of Flt3/Flk2 ligand on mobilization of hematopoietic progenitors in primates and potent synergistic enhancement with granulocyte colony-stimulating factor. Blood. 1997; 90(2):620–629. PMID: 9226162.
Article9. Hosing C, Saliba RM, Ahlawat S, Körbling M, Kebriaei P, Alousi A, De Lima M, Okoroji JG, McMannis J, Qazilbash M, Anderlini P, Giralt S, Champlin RE, Khouri I, Popat U. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009; 84(6):335–337. PMID: 19384931.
Article10. Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010; 45(9):1396–1403. PMID: 20062089.
Article11. Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005; 201(8):1307–1318. PMID: 15837815.
Article12. Cashen AF, Nervi B, DiPersio J. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncol. 2007; 3(1):19–27. PMID: 17280498.
Article13. Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010; 24(5):976–985. PMID: 20357827.
Article14. Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007; 25(12):3093–3100. PMID: 17717064.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of an Ectopic Overexpression of MnSOD in Mouse Hematopoietic Stem Cells
- Rotor Off Fraction Might Contribute Early Platelet Recovery after Syngeneic Transplantation in Mouse
- Expression of adhesion molecules on CD34+ cells from steady-state bone marrow before and after mobilization and their association with the yield of CD34+ cells
- Expression of cytokines and co-stimulatory molecules in the Toxoplasma gondii-infected dendritic cells of C57BL/6 and BALB/c mice
- Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow