J Korean Med Sci.
2012 Dec;27(12):1507-1516. 10.3346/jkms.2012.27.12.1507.
Effect of Small Hairpin RNA Targeting Endothelin-Converting Enzyme-1 in Monocrotaline-Induced Pulmonary Hypertensive Rats
- Affiliations
-
- 1Department of Pediatrics, Konkuk University School of Medicine, Seoul, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Physiology, Konkuk University School of Medicine, Chungju, Korea.
- 4Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. hongym@chollian.net
- KMID: 2157974
- DOI: http://doi.org/10.3346/jkms.2012.27.12.1507
Abstract
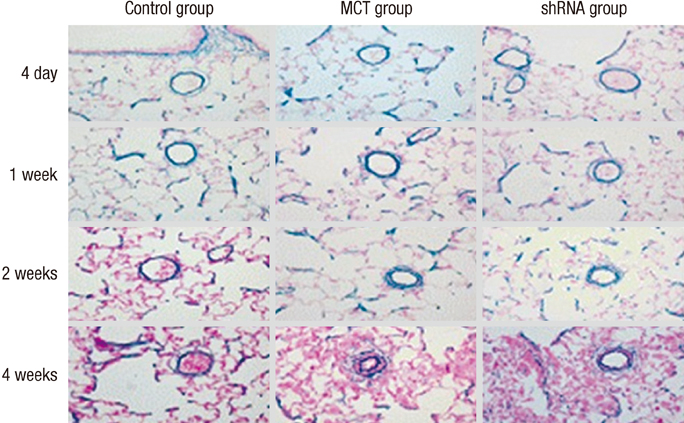
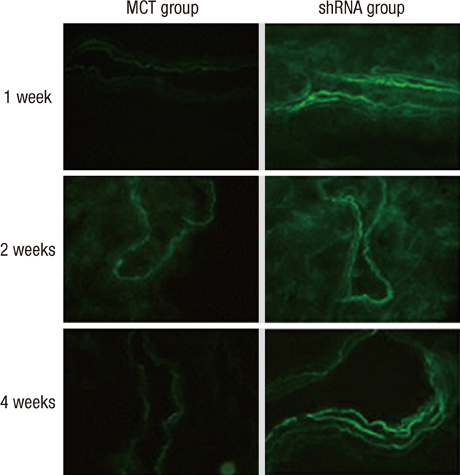
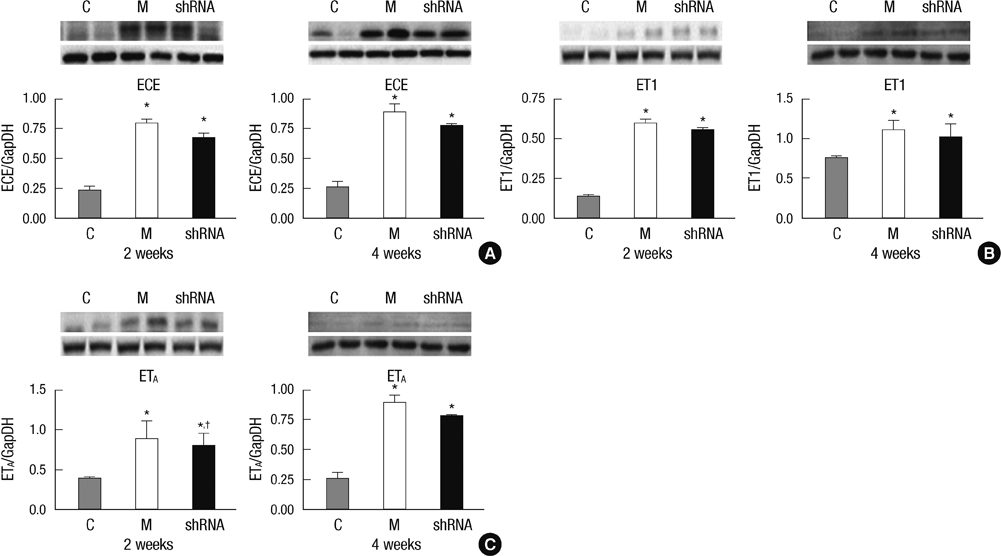
- The purpose of this study was to investigate the therapeutic effects of small hairpin RNA (shRNA) targeting endothelin-converting enzyme (ECE)-1 in monocrotaline (MCT)-induced pulmonary hypertensive rats. Ninty-four Sprague-Dawley rats were divided into three groups: control (n = 24), MCT (n = 35) and shRNA (n = 35). Four-week survival rate in the shRNA group was significantly increased compared to that in the MCT group. The shRNA group showed a significant improvement of right ventricular (RV) pressure compared with the MCT group. The MCT and shRNA groups also showed an increase in RV/(left ventricle + septum) ratio and lung/body weight. Plasma endothelin (ET)-1 concentrations in the shRNA group were lower than those in the MCT group. Medial wall thickness of pulmonary arterioles were increased after MCT injection and was significantly decreased in the shRNA group. The number of intra-acinar muscular pulmonary arteries was decreased in the shRNA group. The mRNA expressions of ET-1 and ET receptor A (ETA) were significantly decreased in the shRNA group in week 4. The protein levels of ETA were decreased in the shRNA group in week 2. The protein levels of tumor necrosis factor-alpha and vascular endothelial growth factor were decreased in the shRNA group in week 4. In conclusion, the gene silencing with lentiviral vector targeting ECE-1 could be effective against hemodynamic, histopathological and gene expression changes in pulmonary hypertension.
Keyword
MeSH Terms
-
Animals
Aspartic Acid Endopeptidases/*antagonists & inhibitors/blood/genetics
Body Weight
Heart Ventricles/physiopathology
Hypertension, Pulmonary/chemically induced/*enzymology/mortality
Lentivirus/genetics
Lung/anatomy & histology/metabolism/pathology
Male
Metalloendopeptidases/*antagonists & inhibitors/blood/genetics
Monocrotaline/toxicity
Pulmonary Artery/drug effects/physiopathology
RNA, Small Interfering/*metabolism
Rats
Rats, Sprague-Dawley
Receptor, Endothelin A/genetics/metabolism
Survival Rate
Tumor Necrosis Factor-alpha/metabolism
Vascular Endothelial Growth Factor A/metabolism
RNA, Small Interfering
Receptor, Endothelin A
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Monocrotaline
Aspartic Acid Endopeptidases
Metalloendopeptidases
Figure
Reference
-
1. Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997. 336:111–117.2. Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003. 361:1533–1544.3. D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Dernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991. 115:343–349.4. Sioud M, Iversen PO. Ribozymes, DNAzymes and small interfering RNAs as therapeutics. Curr Drug Targets. 2005. 6:647–653.5. Puddu GM, Cravero E, Ferrari E, Muscari A, Puddu P. Gene-based therapy for hypertension: do preclinical data suggest a promising future? Cardiology. 2007. 108:40–47.6. Nunez DJR, Brown MJ, Davenport AP, Neylon CB, Schofield JP, Wyse RK. Endothelin-1 messenger-RNA is widely expressed in porcine and human tissues. J Clin Invest. 1990. 85:1537–1541.7. Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duquid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993. 328:1732–1739.8. Yuyama H, Fujimori A, Sanagi M, Koakutsu A, Sudoh K, Sasamata M, Miyata K. The orally active nonpeptide selective endothelin ETA receptor antagonist YM598 prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Eur J Pharmacol. 2004. 496:129–139.9. Liu H, Liu ZY, Guan Q. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact Cardiovasc Thorac Surg. 2007. 6:608–613.10. Hardziyenka M, Campian ME, de Bruin-Bon HA, Michel MC, Tan HL. Sequence of echocardiographic changes during the development of right ventricular failure in rats. J Am Soc Echocardiogr. 2006. 19:1272–1279.11. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010. 40:459–464.12. Altiere RJ, Olson JW, Gillespie MN. Altered pulmonary vascular smooth muscle responsiveness in monocrotaline-induced pulmonary hypertension. J Pharmacol Exp Ther. 1986. 236:390–395.13. Lalich JJ, Merkow L. Pulmonary arteritis produced in rats by feeding Crotalaria spectabilis. Lab Invest. 1961. 10:744–750.14. Campian ME, Hardziyenka M, Michel MC, Tan HL. How valid are animal models to evaluate treatments for pulmonary hypertension? Naunyn Schmiedebergs Arch Pharmacol. 2006. 373:391–400.15. Hill NS, Warburton RR, Pietras L, Klinger JR. Nonspecific endothelin-receptor antagonist blunts monocrotaline-induced pulmonary hypertension in rats. J Appl Physiol. 1997. 83:1209–1215.16. Miyauchi T, Yorikane R, Sakai S, Sakurai T, Okada M, Nishikibe M, Yano M, Yamaguchi I, Sugishita Y, Goto K. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993. 73:887–897.17. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–415.18. Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004. 61:227–237.19. Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994. 344:852–854.20. Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house. Br J Pharmacol. 2008. 153:1105–1119.21. Takahashi M, Fukuda K, Shimada K, Barnes K, Turner AJ, Ikeda M, Koike H, Yamamoto Y, Tanzawa K. Localization of rat endothelin-converting enzyme to vascular endothelial cells and some secretory cells. Biochem J. 1995. 311:657–665.22. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998. 391:806–811.23. Kafri T, Blömer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997. 17:314–317.24. Yang M, Mattes J. Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs. Pharmacol Ther. 2008. 117:94–104.25. Jasmin JF, Lucas M, Cernacek P, Dupuis J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation. 2001. 103:314–318.26. Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med (Maywood). 2006. 231:967–973.27. Kuang T, Wang J, Pang B, Huang X, Burg ED, Yuan JX, Wang C. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm Pharmacol Ther. 2010. 23:456–464.28. Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention hypertension. Pharmacol Ther. 2001. 92:1–20.29. Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995. 269:H1186–H1194.30. Hu LM, Geggel R, Davies P, Reid L. The effect of heparin on the haemodynamic and structural response in the rat to acute and chronic hypoxia. Br J Exp Pathol. 1989. 70:113–124.31. Williams BR. Role of the double-stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem Soc Trans. 1997. 25:509–513.32. Manjunath N, Wu H, Subramanya S, Shankar P. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009. 61:732–745.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Small Hairpin RNA Molecules Targeting Angiotensin-converting Enzyme Gene in Spontaneously Hypertensive Rats
- An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model
- Therapeutic effect of prostaglandin E1 in monocrotaline-induced pulmonary arterial hypertension rats
- Gene Expression of Endothelin-1 and Endothelin Receptor A on Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Effect of endothelin receptor blockade on monocrotaline-induced pulmonary hypertension in rats