Anat Cell Biol.
2017 Mar;50(1):60-68. 10.5115/acb.2017.50.1.60.
Therapeutic effect of prostaglandin E1 in monocrotaline-induced pulmonary arterial hypertension rats
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Seoul National University Bundang Hospital, Seongnam, Korea. beas100@snu.ac.kr
- 2Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2390427
- DOI: http://doi.org/10.5115/acb.2017.50.1.60
Abstract
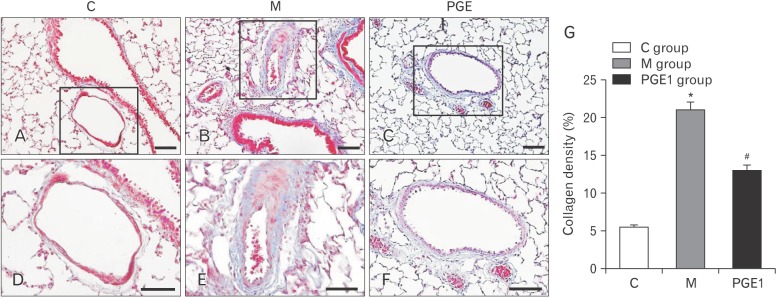
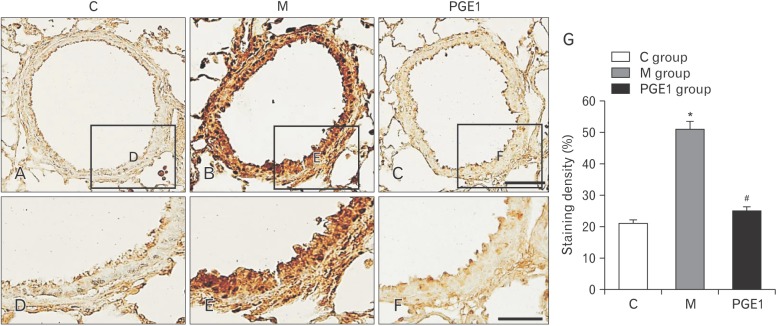
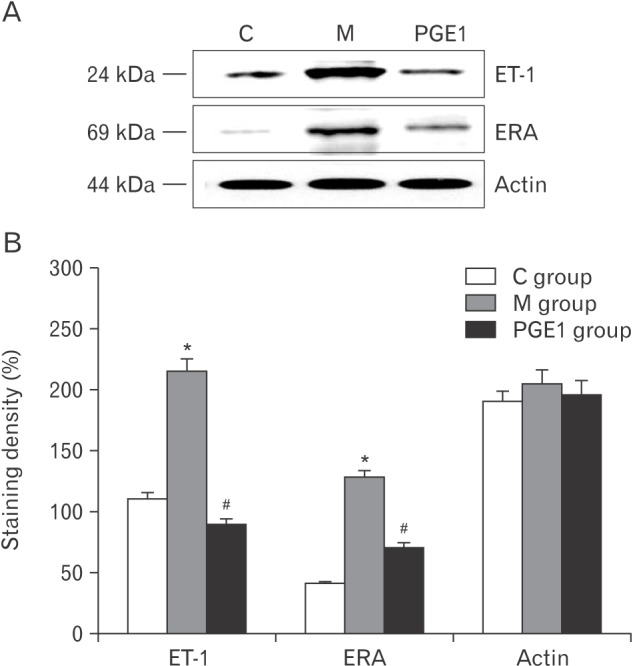
- Pulmonary arterial hypertension (PAH) is a severe pulmonary vascular disease characterized by sustained increase in pulmonary arterial pressure and excessive thickening and remodeling of distal small pulmonary arteries. During disease progression, PAH include increase in mean pulmonary arterial pressure, right ventricular (RV) enlargement, increased pulmonary vascular resistance, and smooth muscle hypertrophy in pulmonary arterioles. Several anti-PAH therapies targeting various pathways involved in PAH progression have been approved by the Food and Drug Adminstration. However, many of the currently available anti-PAH drugs suffer from a number of limitations, including short biological half-life, and poor pulmonary selectivity. Prostaglandin E1 (PGE1) is a potent vasodilator with selectivity toward pulmonary circulation when it is administered via the pulmonary route. However, PGE1 has a very short half-life of 5-10 minutes. Therefore, we hypothesized that long-term effect of PGE1 could reduce mal-adaptive structural remodeling of the lung and heart and prevent ventricular arrhythmias in monocrotaline-induced rat model of PAH. Our results revealed that PGE1 reduced ventricular hypertrophy, protein expressions of endothelin-1 and endothelin receptor A, and the expression of fibrosis. These results support the notion that PGE1 can improve the functional properties of RV, highlighting its potential benefits for heart and lung impairment.
MeSH Terms
-
Alprostadil*
Animals
Arrhythmias, Cardiac
Arterial Pressure
Arterioles
Disease Progression
Endothelin-1
Fibrosis
Half-Life
Heart
Heart Ventricles
Hypertension*
Hypertrophy
Lung
Models, Animal
Muscle, Smooth
Pulmonary Artery
Pulmonary Circulation
Rats*
Receptors, Endothelin
Vascular Diseases
Vascular Resistance
Alprostadil
Endothelin-1
Receptors, Endothelin
Figure
Reference
-
1. Liles JT, Hoyer K, Oliver J, Chi L, Dhalla AK, Belardinelli L. Ranolazine reduces remodeling of the right ventricle and provoked arrhythmias in rats with pulmonary hypertension. J Pharmacol Exp Ther. 2015; 353:480–489. PMID: 25770134.2. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L1013–L1032. PMID: 19748998.3. Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation. 2008; 118:1486–1495. PMID: 18824655.4. Lau EM, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol. 2015; 12:143–155. PMID: 25421168.5. Voelkel NF, Bogaard HJ, Gomez-Arroyo J. The need to recognize the pulmonary circulation and the right ventricle as an integrated functional unit: facts and hypotheses (2013 Grover Conference series). Pulm Circ. 2015; 5:81–89. PMID: 25992273.6. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002; 347:161–167. PMID: 12124404.7. Stanek B, Frey B, Hülsmann M, Berger R, Sturm B, Strametz-Juranek J, Bergler-Klein J, Moser P, Bojic A, Hartter E, Pacher R. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol. 2001; 38:436–442. PMID: 11499735.8. Kloeze J. Relationship between chemical structure and platelet-aggregation activity of prostaglandins. Biochim Biophys Acta. 1969; 187:285–292. PMID: 4981666.9. Sood BG, Delaney-Black V, Aranda JV, Shankaran S. Aerosolized PGE1: a selective pulmonary vasodilator in neonatal hypoxemic respiratory failure results of a Phase I/II open label clinical trial. Pediatr Res. 2004; 56:579–585. PMID: 15295081.10. Wallace JL. Prostaglandins, NSAIDs, and cytoprotection. Gastroenterol Clin North Am. 1992; 21:631–641. PMID: 1516961.11. Della Rocca G, Coccia C, Pompei L, Costa MG, Di Marco P, Pietropaoli P. Inhaled aerosolized prostaglandin E1, pulmonary hemodynamics, and oxygenation during lung transplantation. Minerva Anestesiol. 2008; 74:627–633. PMID: 18971891.12. Igarashi R, Takenaga M, Takeuchi J, Kitagawa A, Matsumoto K, Mizushima Y. Marked hypotensive and blood flow-increasing effects of a new lipo-PGE(1) (lipo-AS013) due to vascular wall targeting. J Control Release. 2001; 71:157–164. PMID: 11274747.13. Nakazawa K, Uchida T, Matsuzawa Y, Yokoyama K, Makita K, Amaha K. Treatment of pulmonary hypertension and hypoxia due to oleic acid induced lung injury with intratracheal prostaglandin E1 during partial liquid ventilation. Anesthesiology. 1998; 89:686–692. PMID: 9743406.14. Sakuma F, Miyata M, Kasukawa R. Suppressive effect of prostaglandin E1 on pulmonary hypertension induced by monocrotaline in rats. Lung. 1999; 177:77–88. PMID: 9929405.15. de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, Keshavjee S. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation. 2001; 72:1505–1512. PMID: 11707737.16. Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Hashimoto S, Maehara Y, Kuwano H. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int. 2005; 18:923–928. PMID: 16008741.17. Golub M, Zia P, Matsuno M, Horton R. Metabolism of prostaglandins A1 and E1 in man. J Clin Invest. 1975; 56:1404–1410. PMID: 1202078.18. Totsuka E, Sasaki M, Takahashi K, Toyoki Y, Seino K, Chiba S, Narumi S, Hakamada K, Morita T, Konn M. The effects of intraportal prostaglandin E1 administration on hepatic warm ischemia and reperfusion injury in dogs. Surg Today. 1995; 25:421–428. PMID: 7640470.19. Sato T, Kato T, Kurokawa T, Yasui O, Hiroshi N, Miyazawa H, Asanuma Y, Koyama K. Continuous infusion of prostaglandin E1 via the superior mesenteric artery can prevent hepatic injury in hepatic artery interruption through passive portal oxygenation. Liver. 2000; 20:179–183. PMID: 10847488.20. Shen J, He B, Wang B. Effects of lipo-prostaglandin E1 on pulmonary hemodynamics and clinical outcomes in patients with pulmonary arterial hypertension. Chest. 2005; 128:714–719. PMID: 16100159.21. Gaskill HV 3rd. Intraportal prostaglandin E1 ameliorates the toxicity of intraportal 2′-deoxy-5-fluorouridine in rats. J Surg Res. 1987; 43:128–132. PMID: 2957549.22. Kahky MP, Lind DS, Cruz AB Jr, Gaskill HV 3rd. Prostaglandin E1 enhances tumoricidal activity of 5-fluoro-2′-deoxyuridine in rats. J Surg Res. 1991; 51:119–123. PMID: 1830914.23. Lee JC, Kim KC, Choe SY, Hong YM. Reduced immunoreactivities of B-type natriuretic peptide in pulmonary arterial hypertension rats after ranolazine treatment. Anat Cell Biol. 2016; 49:7–14. PMID: 27051563.24. Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007; 76:8–18. PMID: 17617392.25. Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004; 43(12 Suppl S):62S–67S. PMID: 15194180.26. Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011; 63:504–511. PMID: 21419223.27. Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001; 358:1119–1123. PMID: 11597664.28. Gupta V, Rawat A, Ahsan F. Feasibility study of aerosolized prostaglandin E1 microspheres as a noninvasive therapy for pulmonary arterial hypertension. J Pharm Sci. 2010; 99:1774–1789. PMID: 19894275.29. Kramm T, Eberle B, Guth S, Mayer E. Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg. 2005; 28:882–888. PMID: 16242948.30. Alhussin W, Verklan MT. Complications of long-term prostaglandin E1 use in newborns with ductal-dependent critical congenital heart disease. J Perinat Neonatal Nurs. 2016; 30:73–79. PMID: 26813395.31. Raz A, Minkes MS, Needleman P. Endoperoxides and thromboxanes: structural determinants for platelet aggregation and vasoconstriction. Biochim Biophys Acta. 1977; 488:305–311. PMID: 560869.32. Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. 1998; 128:1411–1414. PMID: 9732298.33. Zurier RB, Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971; 234:304–305. PMID: 5003042.34. Salvatori R, Guidon PT Jr, Rapuano BE, Bockman RS. Prostaglandin E1 inhibits collagenase gene expression in rabbit synoviocytes and human fibroblasts. Endocrinology. 1992; 131:21–28. PMID: 1377121.35. Levin G, Duffin KL, Obukowicz MG, Hummert SL, Fujiwara H, Needleman P, Raz A. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002; 365(Pt 2):489–496. PMID: 11939906.36. Moncada S, Higgs EA. Metabolism of arachidonic acid. Ann N Y Acad Sci. 1988; 522:454–463. PMID: 3132072.37. Stone DM, Frattarelli DA, Karthikeyan S, Johnson YR, Chintala K. Altered prostaglandin E1 dosage during extracorporeal membrane oxygenation in a newborn with ductal-dependent congenital heart disease. Pediatr Cardiol. 2006; 27:360–363. PMID: 16565906.38. Wright DH, Abran D, Bhattacharya M, Hou X, Bernier SG, Bouayad A, Fouron JC, Vazquez-Tello A, Beauchamp MH, Clyman RI, Peri K, Varma DR, Chemtob S. Prostanoid receptors: ontogeny and implications in vascular physiology. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R1343–R1360. PMID: 11641101.39. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999; 79:1193–1226. PMID: 10508233.40. Meyer J, Theilmeier G, Van Aken H, Bone HG, Busse H, Waurick R, Hinder F, Booke M. Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg. 1998; 86:753–758. PMID: 9539597.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxic pulmonary vasoconstriction and vascular contractility in monocrotaline-induced pulmonary arterial hypertensive rats
- A Morphological Study of the Pulmonary Endothelium and Neuroendocrine Cells in Monocrotaline-Induced Pulmonary Arterial Hypertension
- Retraction notice to “Therapeutic effect of prostaglandin E1 in monocrotaline-induced pulmonary arterial hypertension rats†by Lee JC (Anat Cell Biol 2017;50:60-8)
- Simvastatin, Sildenafil and Their Combination in Monocrotaline Induced Pulmonary Arterial Hypertension
- Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor