J Korean Med Sci.
2012 Nov;27(11):1300-1307. 10.3346/jkms.2012.27.11.1300.
Human Amniotic Fluid Stem Cell-derived Muscle Progenitor Cell Therapy for Stress Urinary Incontinence
- Affiliations
-
- 1Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu, Korea. tgkwon@knu.ac.kr
- 2Department of Urology, CHA Gumi Medical Center, CHA University, Gumi, Korea.
- 3Department of Pathology, Kyungpook National University Hospital, Daegu, Korea.
- 4Department of Urology, Kyungpook National University Hospital, Daegu, Korea.
- 5Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
- KMID: 2157938
- DOI: http://doi.org/10.3346/jkms.2012.27.11.1300
Abstract
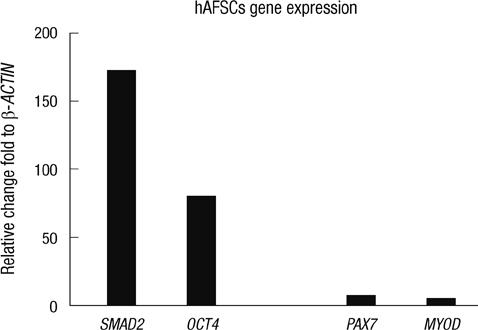
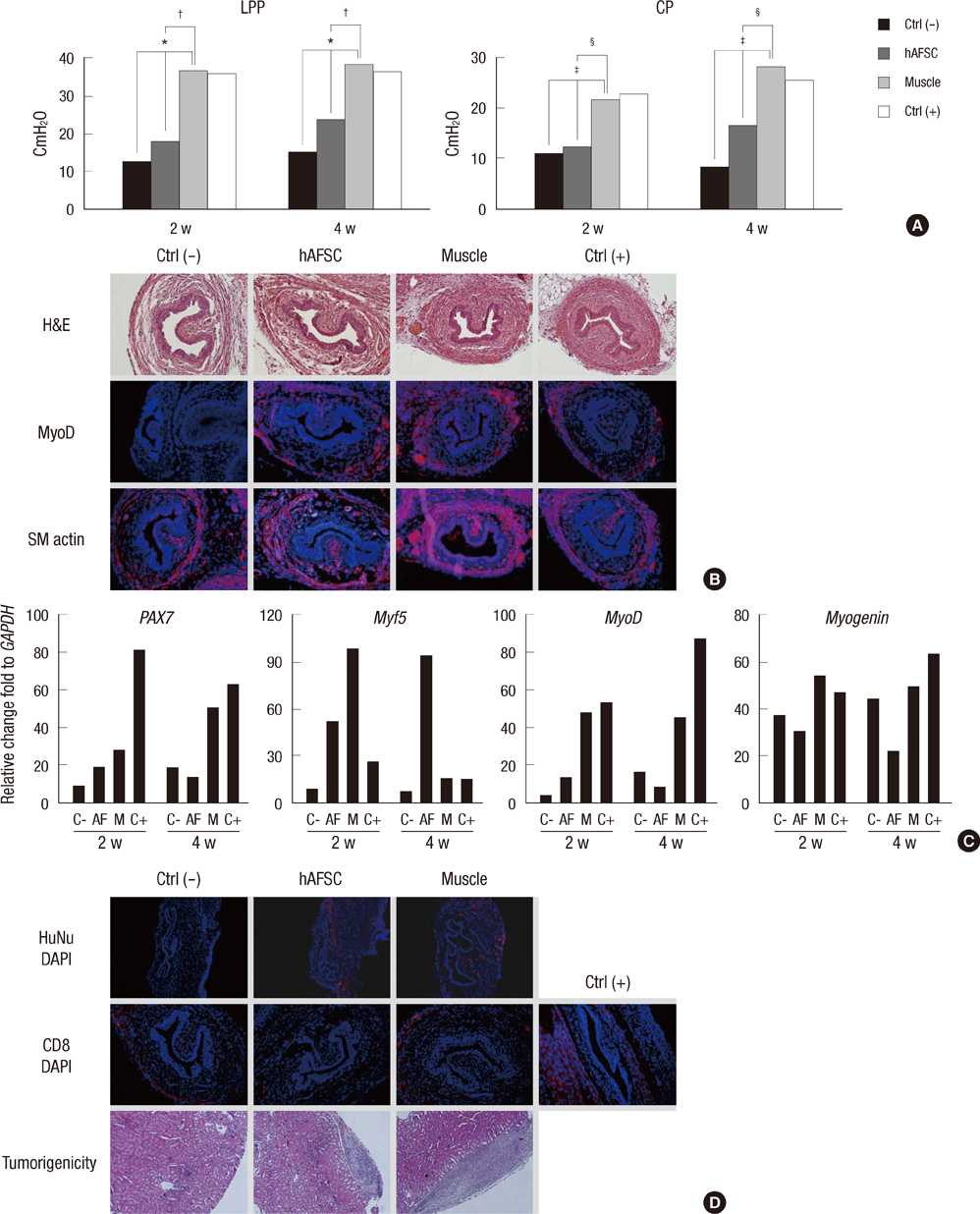
- The most promising treatment for stress urinary incontinence can be a cell therapy. We suggest human amniotic fluid stem cells (hAFSCs) as an alternative cell source. We established the optimum in vitro protocol for the differentiation from hAFSCs into muscle progenitors. These progenitors were transplanted into the injured urethral sphincter and their therapeutic effect was analyzed. For the development of an efficient differentiation system in vitro, we examined a commercial medium, co-culture and conditioned medium (CM) systems. After being treated with CM, hAFSCs were effectively developed into a muscle lineage. The progenitors were integrated into the host urethral sphincter and the host cell differentiation was stimulated in vivo. Urodynamic analysis showed significant increase of leak point pressure and closing pressure. Immunohistochemistry revealed the regeneration of circular muscle mass with normal appearance. Molecular analysis observed the expression of a larger number of target markers. In the immunogenicity analysis, the progenitor group had a scant CD8 lymphocyte. In tumorigenicity, the progenitors showed no teratoma formation. These results suggest that hAFSCs can effectively be differentiated into muscle progenitors in CM and that the hAFSC-derived muscle progenitors are an accessible cell source for the regeneration of injured urethral sphincter.
Keyword
MeSH Terms
-
Amniotic Fluid/*cytology
Animals
Biological Markers/metabolism
Cell Differentiation
Cell Lineage
Cell Transformation, Neoplastic
Cells, Cultured
Coculture Techniques
Female
Gene Expression Regulation
Humans
Immunohistochemistry
Mice
Mice, Inbred ICR
Regeneration
*Stem Cell Transplantation
Stem Cells/*cytology/metabolism
Urethra/physiology
Urinary Incontinence, Stress/pathology/*therapy
Urodynamics
Biological Markers
Figure
Cited by 1 articles
-
Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence
Jae Young Choi, So Young Chun, Bum Soo Kim, Hyun Tae Kim, Eun Sang Yoo, Yun-Hee Shon, Jeong Ok Lim, Seok Joong Yun, Phil Hyun Song, Sung Kwang Chung, James J Yoo, Tae Gyun Kwon
Yonsei Med J. 2015;56(3):648-657. doi: 10.3349/ymj.2015.56.3.648.
Reference
-
1. Deng DY. Urinary incontinence in women. Med Clin North Am. 2011. 95:101–109.2. Sassani P, Aboseif SR. Stress urinary incontinence in women. Curr Urol Rep. 2009. 10:333–337.3. Madjar S, Sharma AK, Waltzer WC, Frischer Z, Secrest CL. Periurethral mass formations following bulking agent injection for the treatment of urinary incontinence. J Urol. 2006. 175:1408–1410.4. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.5. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.6. Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev. 2012. 21:834–843.7. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007. 25:100–106.8. Gekas J, Walther G, Skuk D, Bujold E, Harvey I, Bertrand OF. In vitro and in vivo study of human amniotic fluid-derived stem cell differentiation into myogenic lineage. Clin Exp Med. 2010. 10:1–6.9. Bollini S, Pozzobon M, Nobles M, Riegler J, Dong X, Piccoli M, Chiavegato A, Price AN, Ghionzoli M, Cheung KK, et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011. 7:364–380.10. Yeh YC, Wei HJ, Lee WY, Yu CL, Chang Y, Hsu LW, Chung MF, Tsai MS, Hwang SM, Sung HW. Cellular cardiomyoplasty with human amniotic fluid stem cells: in vitro and in vivo studies. Tissue Eng Part A. 2010. 16:1925–1936.11. Zhang XH, Zeng ZP, Li HZ, Zhou YR, Zhang J, Tong AL, Yan ZL. Expression of renin-angiotensin-aldosterone system in human adipose tissues. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006. 28:766–769.12. Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, Chancellor MB. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004. 23:166–171.13. Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008. 26:2902–2911.14. Cipriani S, Bonini D, Marchina E, Balgkouranidou I, Caimi L, Grassi Zucconi G, Barlati S. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. 2007. 31:845–850.15. Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010. 20:233–243.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence
- Stem Cell Therapy for Erectile Dysfunction
- Use of Stem Cell in Fetal Therapy: Current Status and Future Perspectives
- Human Cord Blood Stem Cell Therapy for Treatment of Stress Urinary Incontinence
- Progenitor Cells in Healing after Pterygium Excision