Yonsei Med J.
2015 May;56(3):648-657. 10.3349/ymj.2015.56.3.648.
Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence
- Affiliations
-
- 1Department of Urology, School of Medicine, Kyungpook National University, Daegu, Korea. tgkwon@knu.ac.kr
- 2Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 3Department of Urology, College of Medicine, Chungbuk National University, Cheongju, Korea.
- 4Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea.
- 5Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
- KMID: 2450337
- DOI: http://doi.org/10.3349/ymj.2015.56.3.648
Abstract
- PURPOSE
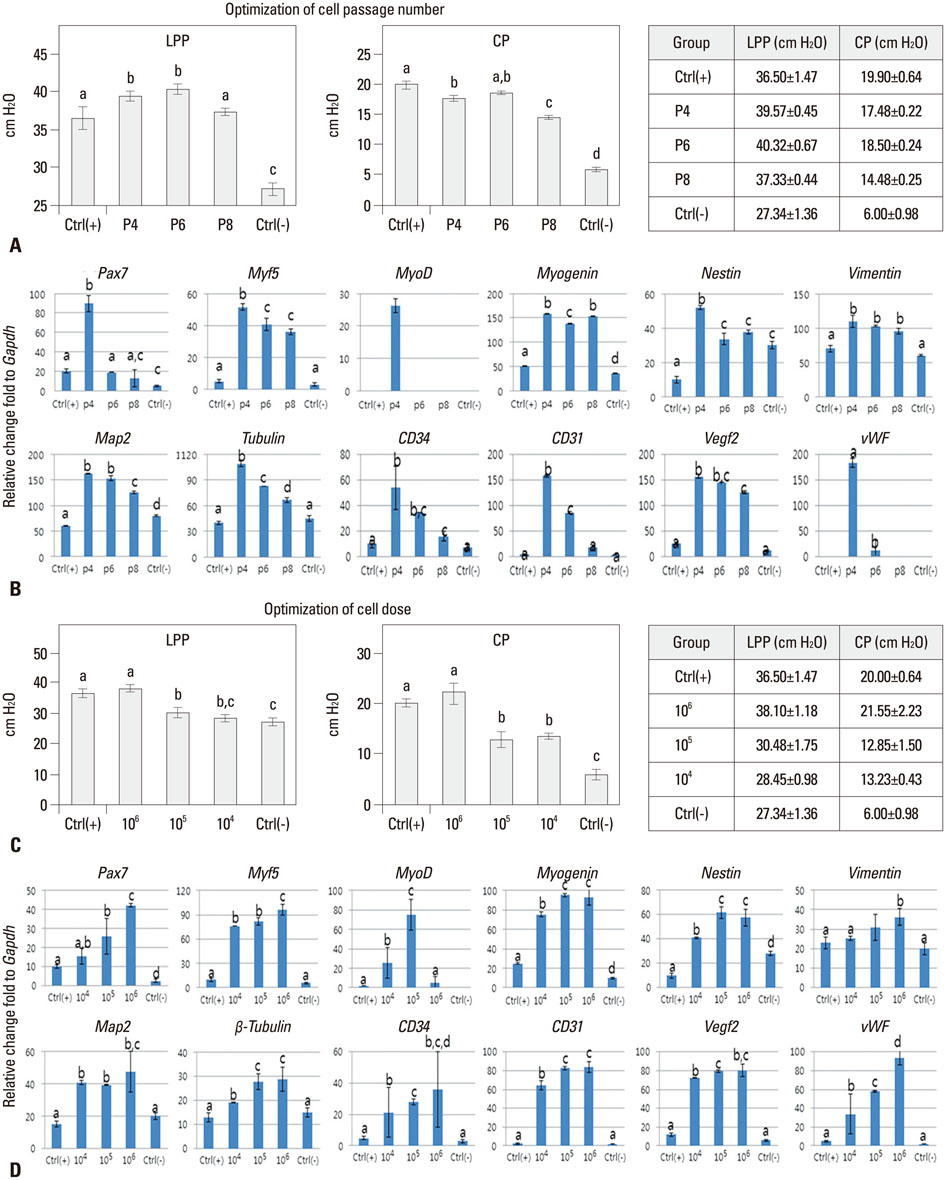
Stem cell-based therapies represent new promises for the treatment of urinary incontinence. This study was performed to assess optimized cell passage number, cell dose, therapeutic efficacy, feasibility, toxicity, and cell trafficking for the first step of the pre-clinical evaluation of human amniotic fluid stem cell (hAFSC) therapy in a urinary incontinence animal model.
MATERIALS AND METHODS
The proper cell passage number was analyzed with hAFSCs at passages 4, 6, and 8 at week 2. The cell dose optimization included 1x10(4), 1x10(5), and 1x10(6) cells at week 2. The in vivo cell toxicity was performed with 0.25x10(6), 0.5x10(6), and 1x10(6) cells at weeks 2 and 4. Cell tracking was performed with 1x10(6) cells at weeks 2 and 4.
RESULTS
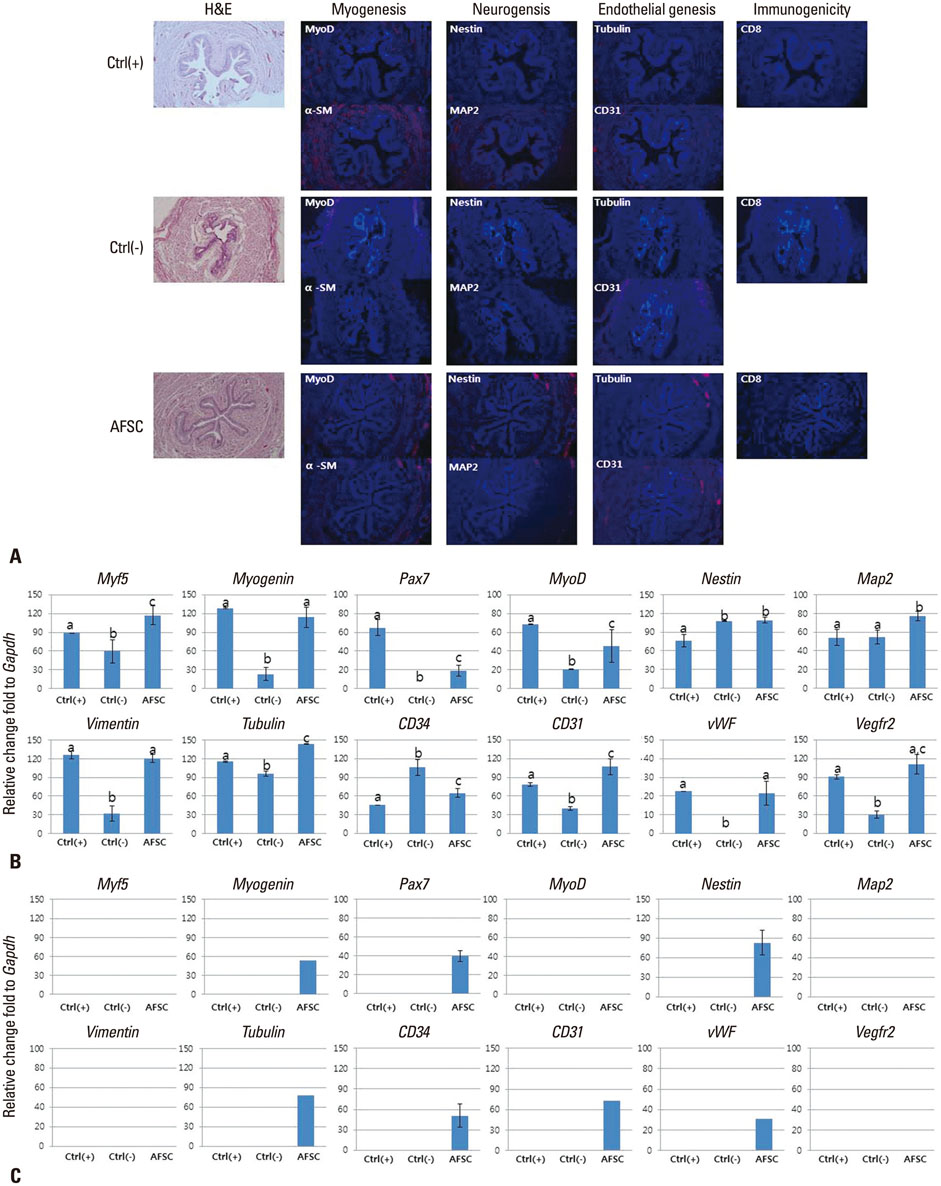
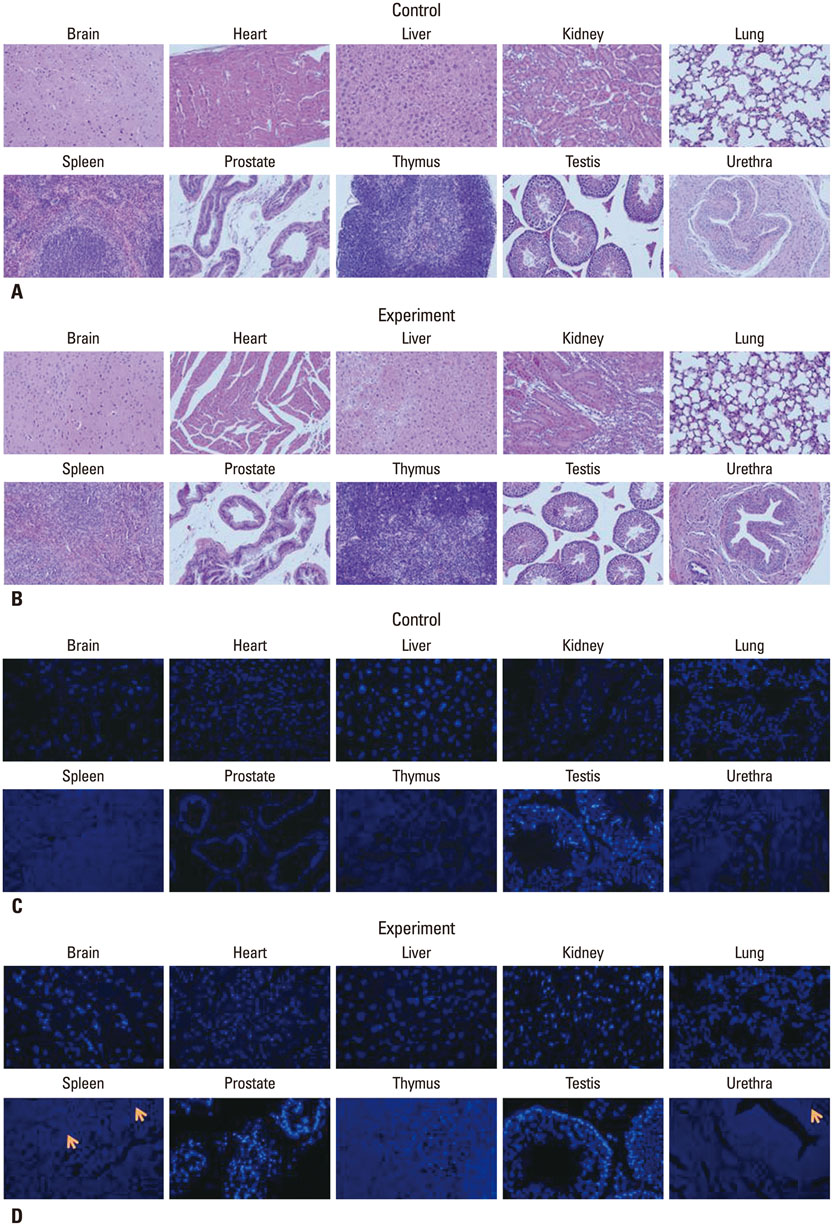
The selected optimal cell passage number was smaller than 6, and the optimal cell dose was 1x10(6) for the mouse model. In our pre-clinical study, hAFSC-injected animals showed normal values for several parameters. Moreover, the injected cells were found to be non-toxic and non-tumorigenic. Furthermore, the injected hAFSCs were rarely identified by in vivo cell trafficking in the target organs at week 2.
CONCLUSION
This study demonstrates for the first time the pre-clinical efficacy and safety of hAFSC injection in the urinary incontinence animal model and provides a basis for future clinical applications.
MeSH Terms
Figure
Reference
-
1. Sassani P, Aboseif SR. Stress urinary incontinence in women. Curr Urol Rep. 2009; 10:333–337.
Article2. Kim SO, Na HS, Kwon D, Joo SY, Kim HS, Ahn Y. Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int. 2011; 86:110–116.
Article3. Kim BS, Chun SY, Lee JK, Lim HJ, Bae JS, Chung HY, et al. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012; 10:94.
Article4. Chun SY, Cho DH, Chae SY, Choi KH, Lim HJ, Yoon GS, et al. Human amniotic fluid stem cell-derived muscle progenitor cell therapy for stress urinary incontinence. J Korean Med Sci. 2012; 27:1300–1307.
Article5. Kim H, Kim HY, Choi MR, Hwang S, Nam KH, Kim HC, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010; 468:190–194.
Article6. Morita E, Watanabe Y, Ishimoto M, Nakano T, Kitayama M, Yasui K, et al. A novel cell transplantation protocol and its application to an ALS mouse model. Exp Neurol. 2008; 213:431–438.
Article7. Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000; 28:707–715.
Article8. Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997; 64:278–294.
Article9. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726–736.
Article10. Rocha V, Labopin M, Gluckman E, Powles R, Arcese W, Bacigalupo A, et al. Relevance of bone marrow cell dose on allogeneic transplantation outcomes for patients with acute myeloid leukemia in first complete remission: results of a European survey. J Clin Oncol. 2002; 20:4324–4330.
Article11. Hohaus S, Wallwiener D, Martin S, Voso MT, Huober J, Fersis N, et al. Efficacy and toxicity of sequential high-dose therapy with peripheral blood stem cell support in patients with high-risk breast cancer. Semin Oncol. 1998; 25:2 Suppl 4. 7–11.12. Plitnick LM, Herzyk DJ. Nonclinical development of novel biologics, biosimilars, vaccines and specialty biologics. Amsterdam: Academic Press;2013. p. 388–390.13. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007; 25:100–106.
Article14. Choi MR, Kim HY, Park JY, Lee TY, Baik CS, Chai YG, et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2010; 472:94–98.
Article15. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999; 107:275–281.
Article16. Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996; 166:585–592.
Article17. Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005; 32:270–279.
Article18. Faustman DL, Davis M. Stem cells in the spleen: therapeutic potential for Sjogren's syndrome, type I diabetes, and other disorders. Int J Biochem Cell Biol. 2010; 42:1576–1579.
Article19. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–3506.
Article20. Itoi M, Tsukamoto N, Yoshida H, Amagai T. Mesenchymal cells are required for functional development of thymic epithelial cells. Int Immunol. 2007; 19:953–964.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Amniotic Fluid Stem Cell-derived Muscle Progenitor Cell Therapy for Stress Urinary Incontinence
- Human Cord Blood Stem Cell Therapy for Treatment of Stress Urinary Incontinence
- Effects of Injection Therapy using Muscle Derived Stem Cell/Chitosan/Hydroapatite Composite Gel in a Rat Model of Urinary Incontinence
- Problems Associated with Establishment of Human Embryonic Stem Cell
- A study on in vitro developmental promoting effect of pronucleate I-cell mouse embryos by human amniotic fluid