Yonsei Med J.
2007 Feb;48(1):48-54. 10.3349/ymj.2007.48.1.48.
Progenitor Cells in Healing after Pterygium Excision
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Chung-Ang University, Seoul, Korea. jck50ey@kornet.net
- KMID: 1093523
- DOI: http://doi.org/10.3349/ymj.2007.48.1.48
Abstract
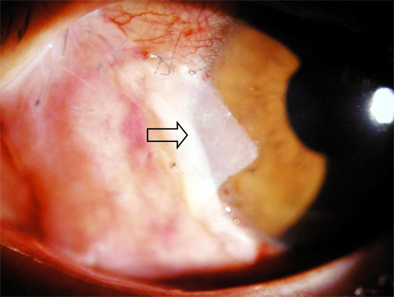
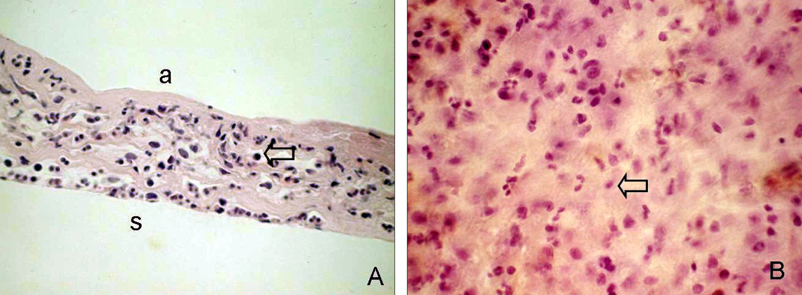
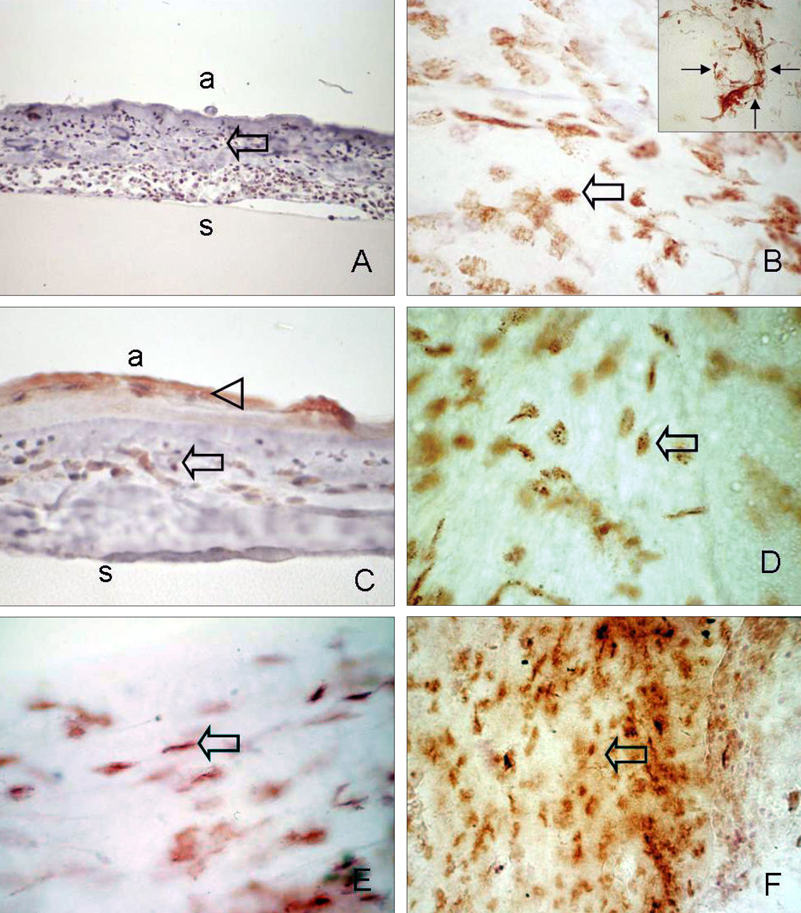
- Bone marrow derived progenitor cells were reported to be involved in the pathogenesis of pterygium and have been suggested to be important in angiogenesis and the repair process after tissue damage. In order to investigate the involvement of these cells in wound healing after a pterygium excision, immunohistochemical staining was performed with a temporary amniotic membrane, applied to the bare sclera, after a pterygium excision using various progenitor cell markers, including CD34, c-kit, STRO-1, and AC133, to determine the expression levels of the participating cells. CD34-positive cells were observed along with some round or spindle-shaped mononuclear cells on the stromal side of the amniotic membrane. Some CD34-positive, large, and round or spindle-shaped cells formed clusters resembling small vessels in some regions of the amniotic membrane. c-kit was expressed in the epithelium that had grown over the amniotic membrane and in the spindle-shaped or round mononuclear cells in the stroma. Many stellate- to spindle-shaped fibroblast like cells expressed STRO-1, and AC133 was expressed in some round and ovoid cells. Overall, these results suggest that adult bone marrow- derived progenitor cells, such as endothelial progenitor cells and mesenchymal stem cells, are involved in the wound healing process post-excision in patients with pterygium.
Keyword
Figure
Reference
-
1. Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999. 10:282–288.
Article2. Kwok LS, Coroneo MT. A model for pterygium formation. Cornea. 1994. 13:219–224.
Article3. Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997. 38:2483–2491.4. Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002. 25:17–22.
Article5. Nakagami T, Watanabe I, Murakami A, Okisaka S, Ebihara N. Expression of stem cell factor in pterygium. Jpn J Ophthalmol. 2000. 44:193–197.
Article6. Ye J, Song YS, Kang SH, Yao K, Kim JC. Involvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygium. Eye. 2004. 18:839–843.
Article7. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
Article8. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004. 172:1266–1272.
Article9. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.
Article10. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999. 103:697–705.
Article11. Boyd AW. Human leukocyte antigens: an update on structure, function, and nomenclature. Pathology. 1987. 19:329–337.
Article12. Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994. 84:691–701.
Article13. Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the S1 locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990. 63:213–224.
Article14. Schmeisser A, Strasser RH. Phenotypic overlap between hematopoietic cells with suggested angioblastic potential vascular endothelial cells. J Hematother Stem Cell Res. 2002. 11:69–79.
Article15. Majka M, Ratajczak J, Machalinski B, Carter A, Pizzini D, Wasik MA, et al. Expression, regulation and function of AC133, a putative cell surface marker of primitive human hematopoietic cells. Folia Histochem Cytobiol. 2000. 38:53–63.16. Simmons PJ, Torok-Strob B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.
Article17. Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000. 41:671–679.18. Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993. 34:1814–1828.19. Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990. 62:690–696.20. Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000. 95:3106–3112.
Article21. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.
Article22. Pompen M, Smids BS, Dingemans KP, Jansen HM, Out TA, Lutter R. Lung epithelial H292 cells induce differentiation of immature human HMC-1 mast cells by interleukin-6 and stem cell factor. Clin Exp Allergy. 2000. 30:1104–1112.
Article23. Sakaguchi H, Takai S, Sakaguchi M, Sugiyama T, Ishihara T, Yao Y, et al. Chymase and angiotensin converting enzyme activities in a hamster model of glaucoma filtering surgery. Curr Eye Res. 2002. 24:325–331.
Article24. Nakagami T, Murakami A, Okisaka S, Ebihara N. Mast cells in pterygium: number and phenotype. Jpn J Ophthalmol. 1999. 43:75–79.
Article25. Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003. 90:570–576.
Article26. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004. 10:858–864.
Article27. Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montano J, Chang H, et al. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999. 93:3379–3390.
Article28. Pelletier L, Angonin R, Regnard J, Fellmann D, Charbord P. Human bone marrow angiogenesis: in vitro modulation by substance P and neurokinin A. Br J Haematol. 2002. 119:1083–1089.
Article29. Park WC, Tseng SC. Modulation of acute inflammation and keratocyte death by suturing, blood, and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000. 41:2906–2914.30. Jang JH, Choi TH. The effect of amniotic membrane transplantation for pterygium excision. J Korean Ophthalmol Soc. 2005. 46:597–604.31. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005. 24:141–150.
Article32. Essex RW, Snibson GR, Daniell M, Tole DM. Amniotic membrane grafting in the surgical management of primary pterygium. Clin Experiment Ophthalmol. 2004. 32:501–504.
Article33. Tananuvat N, Martin T. The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea. 2004. 23:458–463.
Article34. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000. 70:329–337.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating Endothelial Progenitor Cells and Vasculogenic Factors in Pterygium Pathogenesis
- A Simplified way to Remove the Head of Pterygium
- The Effect of Subconjunctival Bevacizumab Injection after Primary Pterygium Surgery
- Comparison of Corneal Higher-order Aberration before and after Excision of Pterygium
- Surgical Outcome of Primary Pterygium Excision with Conjunctival Autograft