Ann Dermatol.
2012 May;24(2):136-143.
Paracrine Effects of Adipose-Derived Stem Cells on Keratinocytes and Dermal Fibroblasts
- Affiliations
-
- 1Department of Dermatology, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Soprano Clinic, Seoul, Korea.
- 3Modelo Clinic, Seoul, Korea.
- 4Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea. khcho@snu.ac.kr
Abstract
- BACKGROUND
Adipose-derived stem cells (ASCs) are mesenchymal stem cells that have recently been applied to tissue repair and regeneration. Keratinocytes and dermal fibroblasts play key roles in cutaneous wound healing.
OBJECTIVE
We investigated the paracrine effects of ASCs on HaCaT cells (i.e., immortalized human keratinocytes) and human dermal fibroblasts to explore the mechanism of the effects of ASCs on cutaneous wound healing.
METHODS
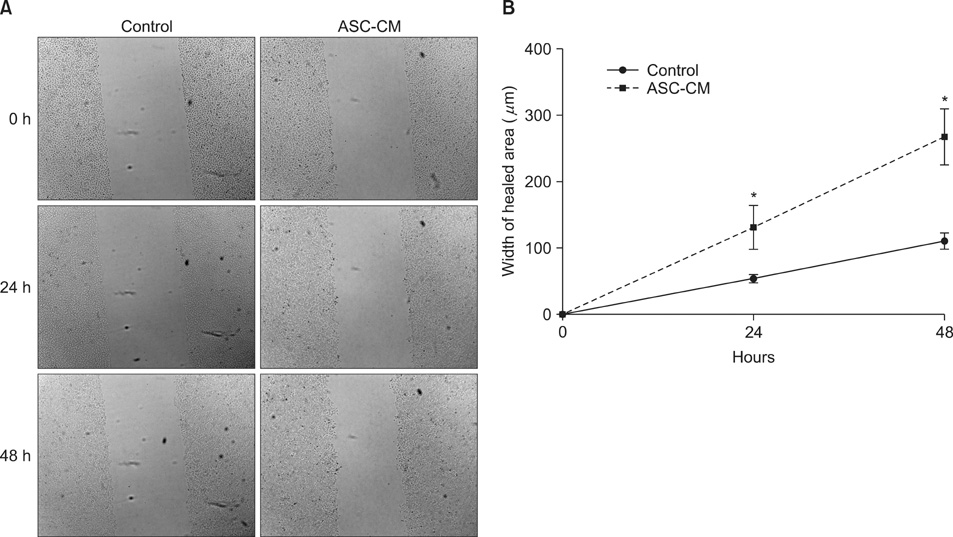
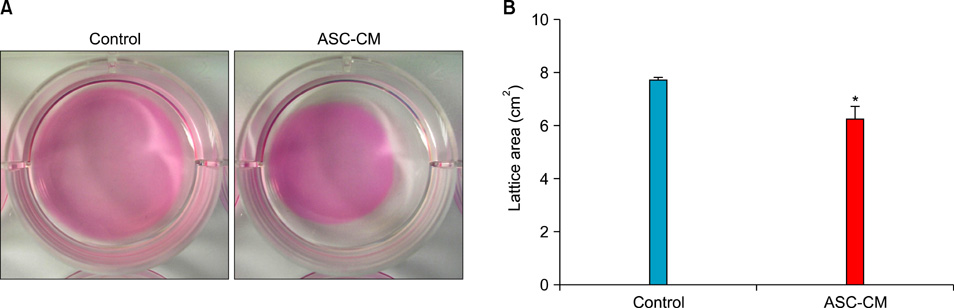
HaCaT cells and primary cultured human dermal fibroblasts were treated with 50% conditioned medium of ASCs (ASC-CM). Viability, in vitro wound healing, and fibroblast-populated collagen lattice contraction assays were conducted, and reverse transcription-polymerase chain reaction (RT-PCR) for the type I procollagen alpha1 chain gene was performed.
RESULTS
The proliferation of HaCaT cells and fibroblasts was increased by ASC-CM in the viability assay. ASC-CM promoted in vitro wound healing of HaCaT cells and increased the contraction of the fibroblast-populated collagen lattice. RT-PCR showed that the transcription of the type I procollagen alpha1 chain gene in fibroblasts was upregulated by ASC-CM.
CONCLUSION
The stimulatory effect of ASC on cutaneous wound healing may be partially mediated by paracrine effects of ASCs on other skin cells. Application of ASCs or ASC-derived molecules could be an innovative therapeutic approach in the treatment of chronic wounds and other conditions.
MeSH Terms
Figure
Reference
-
1. Steeper R. A critical review of the aetiology of diabetic neuropathic ulcers. J Wound Care. 2005. 14:101–103.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article3. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.
Article4. Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001. 37:1726–1732.
Article5. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007. 25:2648–2659.
Article6. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008. 3:e1886.
Article7. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article8. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.
Article9. Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009. 62:317–321.
Article10. Park JS, Park WY, Cho KA, Kim DI, Jhun BH, Kim SR, et al. Down-regulation of amphiphysin-1 is responsible for reduced receptor-mediated endocytosis in the senescent cells. FASEB J. 2001. 15:1625–1627.
Article11. Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006. 17:279–290.
Article12. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999. 341:738–746.
Article13. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009. 17:540–547.
Article14. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007. 48:15–24.
Article15. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005. 31:674–686.
Article16. Sarret Y, Woodley DT, Grigsby K, Wynn K, O'Keefe EJ. Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol. 1992. 98:12–16.
Article17. Bhora FY, Dunkin BJ, Batzri S, Aly HM, Bass BL, Sidawy AN, et al. Effect of growth factors on cell proliferation and epithelialization in human skin. J Surg Res. 1995. 59:236–244.
Article18. Ishimoto S, Ishibashi T, Bottaro DP, Kaga K. Direct application of keratinocyte growth factor, basic fibroblast growth factor and transforming growth factor-alpha during healing of tympanic membrane perforation in glucocorticoid-treated rats. Acta Otolaryngol. 2002. 122:468–473.
Article19. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003. 83:835–870.
Article20. Yamaguchi Y, Crane S, Zhou L, Ochoa SM, Falanga V. Lack of co-ordinate expression of the alpha1(I) and alpha1(III) procollagen genes in fibroblast clonal cultures. Br J Dermatol. 2000. 143:1149–1153.
Article21. Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988. 85:4894–4897.
Article22. Clark RA, Folkvord JM, Hart CE, Murray MJ, McPherson JM. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989. 84:1036–1040.
Article23. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- The Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on the Formation ofEpidermis and Basement Membrane in Artificial Skin Models
- Enhancing the Angiogenic and Proliferative Capacity of Dermal Fibroblasts with Mulberry ( Morus alba. L) Root Extract