Korean J Pain.
2015 Oct;28(4):236-243. 10.3344/kjp.2015.28.4.236.
A New Rat Model of Cisplatin-induced Neuropathic Pain
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School, Gwangju, Korea. mhyoon@chonnam.ac.kr
- 2Center for Creative Biomedical Scientists at Chonnam National University, Gwangju, Korea.
- KMID: 2151666
- DOI: http://doi.org/10.3344/kjp.2015.28.4.236
Abstract
- BACKGROUND
Chemotherapy-induced peripheral neuropathy is a major side effect of anti-cancer drugs, and our knowledge of its mechanisms is lacking. Several models for chemotherapy-induced neuropathy have been introduced. However, the outcomes of these models differ significantly among laboratories. Our object was to create a model of chemotherapy-induced neuropathy in rats with cancer.
METHODS
Female Sprague-Dawley rats were used. Mammary rat metastasis tumor (MRMT-1) cells were implanted subcutaneously in rats. Chemotherapy-induced peripheral neuropathy was induced by injection of cisplatin once a day for four days. The responses to mechanical and thermal stimuli were examined using von Frey filaments, acetone, and radiant heat.
RESULTS
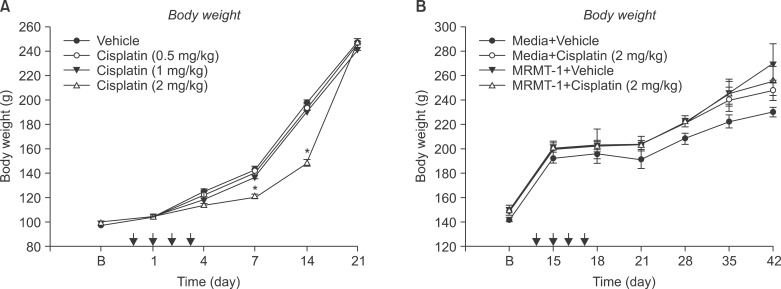
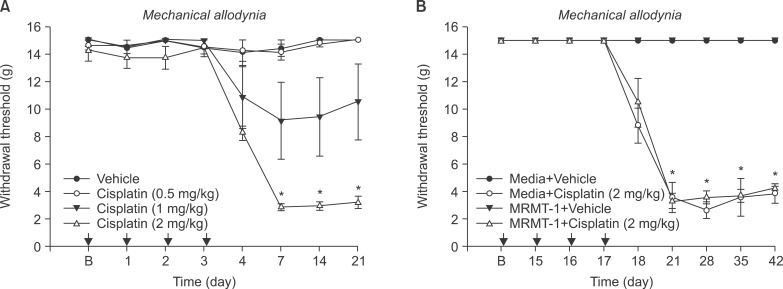
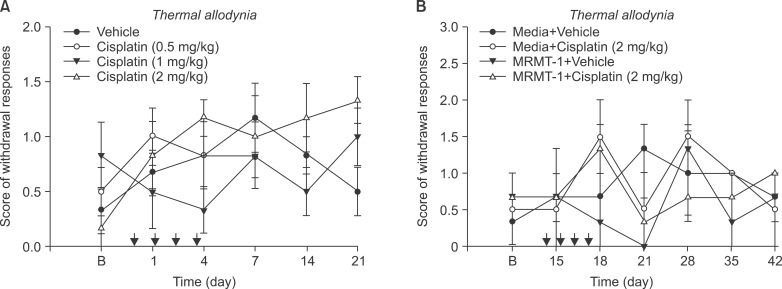
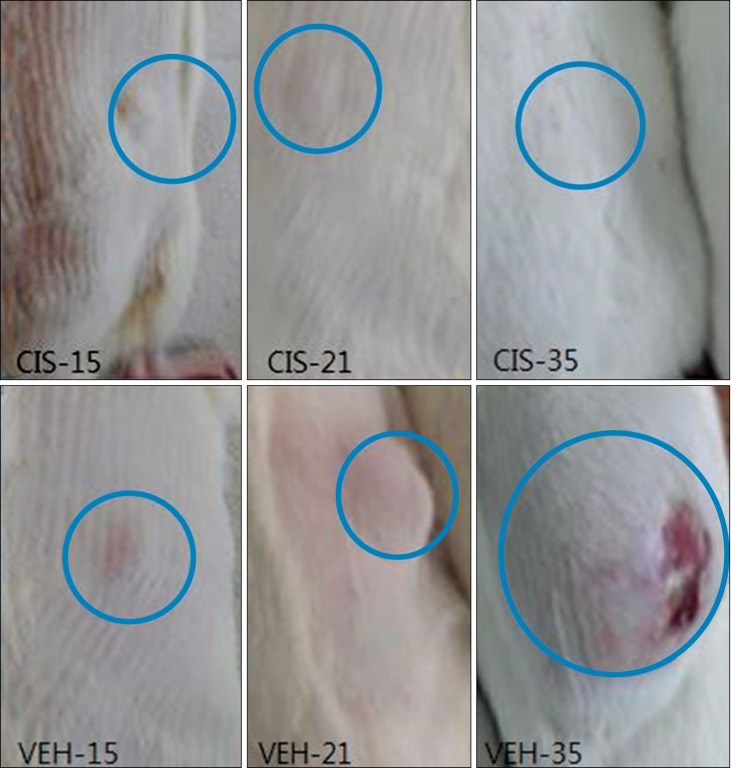
Cisplatin (2 mg/kg/day) produced mechanical allodynia, while it did not induce cold allodynia or thermal hyperalgesia. This dose of cisplatin could work successfully against cancer. Body weight loss was not observed in cisplatin-treated rats, nor were other abnormal behaviors noted in the same rats.
CONCLUSIONS
Repeated injection of intraperitoneal cisplatin induced peripheral neuropathic pain in rats. Thus, this type of rat model has broad applicability in studies related to searching for the mechanism of cisplatin-induced mechanical allodynia and agents for the treatment of neuropathic pain.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Antinociceptive role of neurotensin receptor 1 in rats with chemotherapy-induced peripheral neuropathy
Mei Yin, Yeo-Ok Kim, Jeong-Il Choi, Seongtae Jeong, Si-Ho Yang, Hong-Beom Bae, Myung-Ha Yoon
Korean J Pain. 2020;33(4):318-325. doi: 10.3344/kjp.2020.33.4.318.Prostaglandin D2 contributes to cisplatin-induced neuropathic pain in rats via DP2 receptor in the spinal cord
Yaqun Li, Woong Mo Kim, Seung Hoon Kim, Hyun Eung You, Dong Ho Kang, Hyung Gon Lee, Jeong Il Choi, Myung Ha Yoon
Korean J Pain. 2021;34(1):27-34. doi: 10.3344/kjp.2021.34.1.27.
Reference
-
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008; 13:27–46. PMID: 18346229.
Article2. Höke A. Animal models of peripheral neuropathies. Neurotherapeutics. 2012; 9:262–269. PMID: 22415319.
Article3. Franconi G, Manni L, Schröder S, Marchetti P, Robinson N. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. 2013; 2013:516916. PMID: 23983788.
Article4. Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009; 6:620–629. PMID: 19789067.
Article5. Cavaletti G, Petruccioli MG, Tredici G, Marmiroli P, Barajon I, Fabbrica D, et al. Effects of repeated administration of low doses of cisplatin on the rat nervous system. Int J Tissue React. 1991; 13:151–157. PMID: 1960015.6. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008; 44:1507–1515. PMID: 18571399.
Article7. Jeong S, Lee SH, Kim YO, Yoon MH. Antinociceptive effects of amiloride and benzamil in neuropathic pain model rats. J Korean Med Sci. 2013; 28:1238–1243. PMID: 23960454.
Article8. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–376. PMID: 7708411.
Article9. Marmiroli P, Nicolini G, Miloso M, Scuteri A, Cavaletti G. The fundamental role of morphology in experimental neurotoxicology: the example of chemotherapy-induced peripheral neurotoxicity. Ital J Anat Embryol. 2012; 117:75–97. PMID: 23420996.10. Rostock M, Jaroslawski K, Guethlin C, Ludtke R, Schröder S, Bartsch HH. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evid Based Complement Alternat Med. 2013; 2013:349653. PMID: 24066010.
Article11. Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013; 9:25–33. PMID: 22648527.
Article12. Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2013; 116:224–231. PMID: 23223118.
Article13. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006; 6:836–848. PMID: 17063185.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Anorexia and Neuropathic Pain Induced by Cisplatin on Hindlimb Muscles of Rat
- Development of Neuropathic Pain Model with 1% Phenol Injection in Rat Sciatic Nerve
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain
- The Combined Antiallodynic Effect of Gabapentin and Milnacipran in a Rat Neuropathic Pain Model
- Effects of Clonidine and Yohimbine on Neuropathic Pain in a Rat Model