Immune Netw.
2010 Aug;10(4):135-143. 10.4110/in.2010.10.4.135.
Cordyceps militaris Enhances MHC-restricted Antigen Presentation via the Induced Expression of MHC Molecules and Production of Cytokines
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk University, Cheongju 361-763, Korea.
- 3Department of Biology, Seoul Women's University, Seoul 139-774, Korea.
- KMID: 2150672
- DOI: http://doi.org/10.4110/in.2010.10.4.135
Abstract
- BACKGROUND
Cordyceps militarys water extract (CME) has been reported to exert antitumor and immunomodulatory activities in vivo and in vitro. However, the therapeutic mechanism has not yet been elucidated. In this study, we examined the effects of CME on the antigen presenting function of antigen presenting cells (APCs).
METHODS
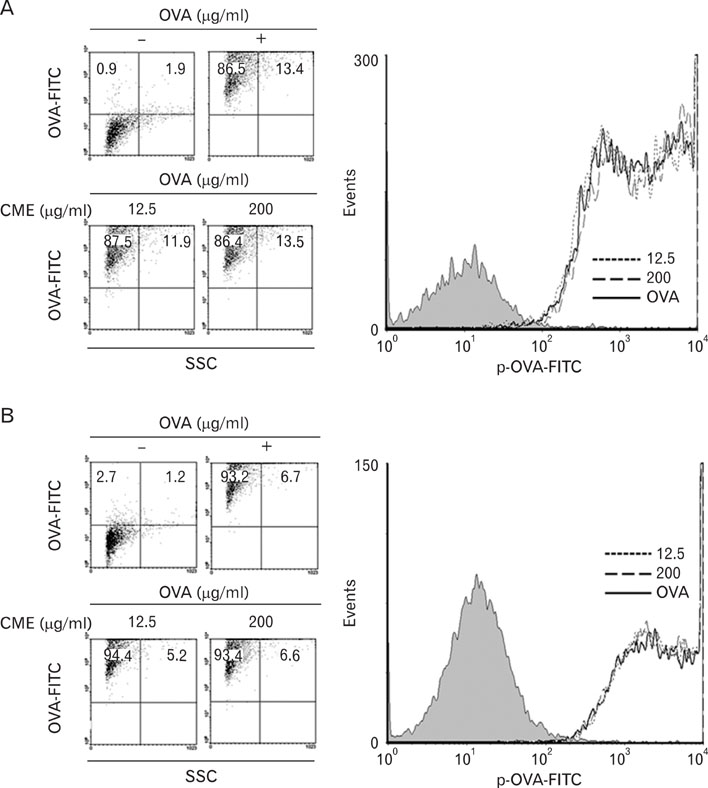
Dendritic cells (DCs) were cultured in the presence of CME, and then allowed to phagocytose microspheres containing ovalbumin (OVA). After washing and fixing the efficacy of OVA, peptide presentation by DCs were evaluated using CD8 and CD4 T cells. Also, we confirmed the protein levels of proinflammatory cytokines through western blot analysis.
RESULTS
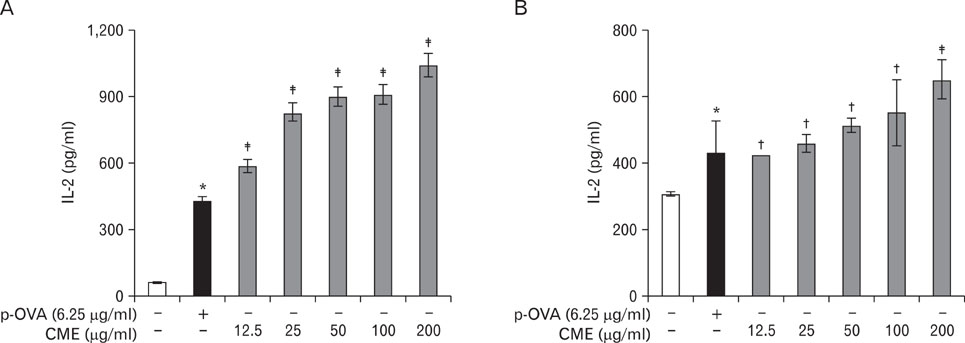
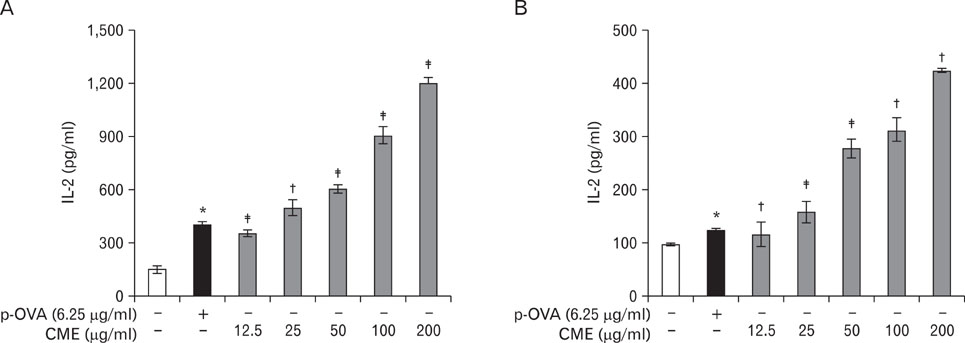
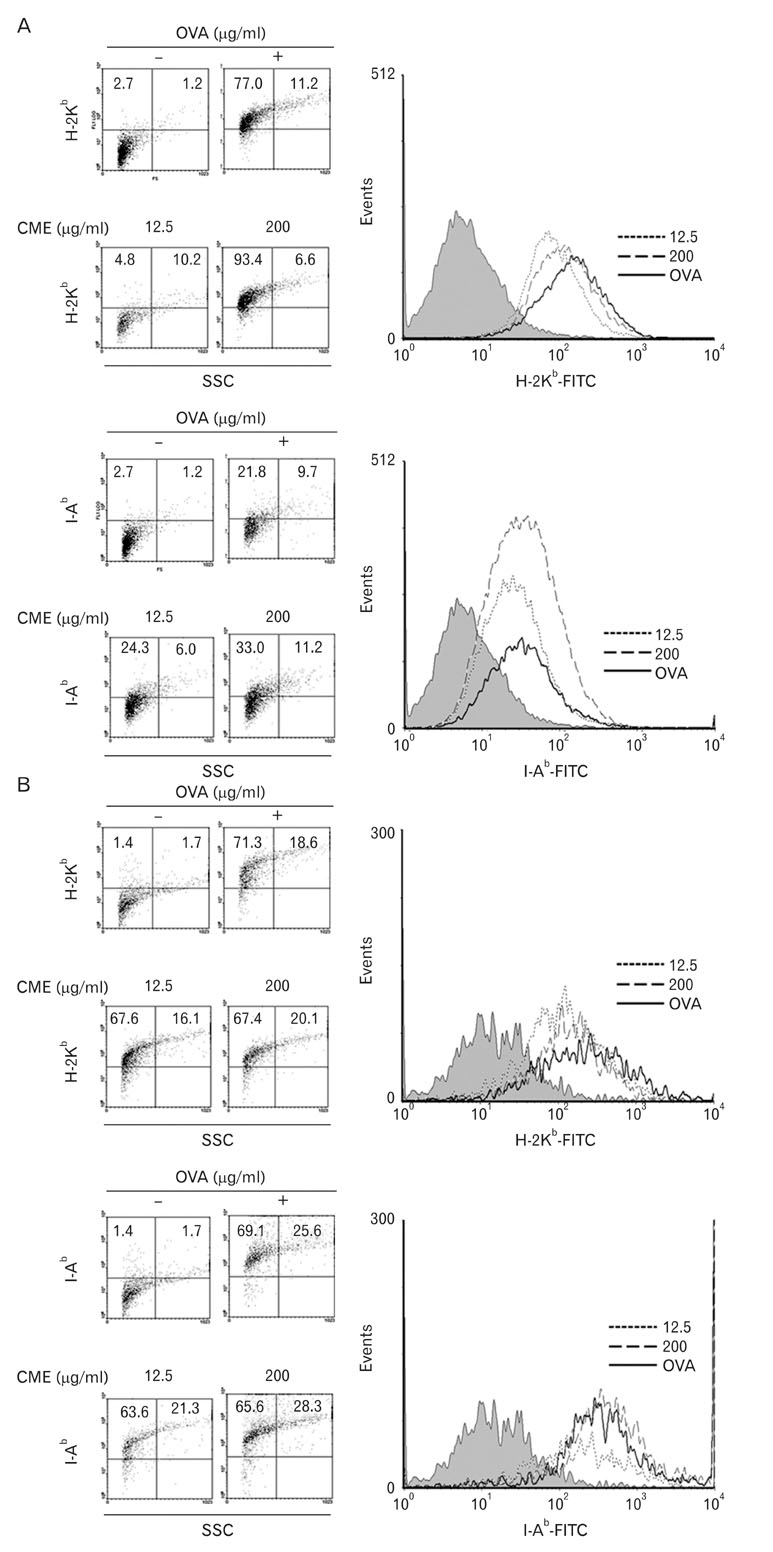
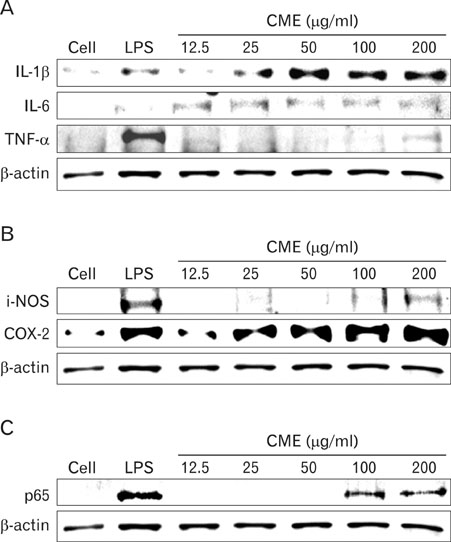
CME enhanced both MHC class I and class II-restricted presentation of OVA in DCs. In addition, the expression of both MHC class I and II molecules was enhanced, but there was no changes in the phagocytic activity of exogenous OVA. Furthermore, CME induced the protein levels of iNOS, COX-2, proinflammatory cytokines, and nuclear p65 in a concentration-dependent manner, as determined by western blot.
CONCLUSION
These results provide an understanding of the mechanism of the immuno-enhancing activity of CME on the induction of MHC-restricted antigen presentation in relation to their actions on APCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhang Y, Kiyohara H, Matsumoto T, Yamada H. Fractionation and chemical properties of immunomodulating polysaccharides from roots of Dipsacus asperoides. Planta Med. 1997. 63:393–399.
Article2. Matsumoto T, Sun XB, Hanawa T, Kodaira H, Ishii K, Yamada H. Effect of the antiulcer polysaccharide fraction from Bupleurum falcatum L. on the healing of gastric ulcer induced by acetic acid in rats. Phytother Res. 2002. 16:91–93.
Article3. Huang LF, Liang YZ, Guo FQ, Zhou ZF, Cheng BM. Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militarris by LC/ESI-MS. J Pharm Biomed Anal. 2003. 33:1155–1162.
Article4. Shin S, Moon S, Park Y, Kwon J, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Role of cordycepin and adenosine on the phenotypic switch of macrophages via induced anti-inflammatory cytokines. Immune Netw. 2009. 9:255–264.
Article5. Yu R, Wang L, Zhang H, Zhou C, Zhao Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia. 2004. 75:662–666.
Article6. Shin S, Kwon J, Lee S, Kong H, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Immunostimulatory effects of cordyceps militaris on macrophages through the enhanced production of cytokines via the activation of NF-kappaB. Immune Netw. 2010. 10:55–63.
Article7. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article8. Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991. 147:2860–2863.9. Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997. 158:2723–2730.10. Lee JK, Lee MK, Yun YP, Kim Y, Kim JS, Kim YS, Kim K, Han SS, Lee CK. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001. 1:1275–1284.
Article11. Lee YH, Lee YR, Im SA, Park SI, Kim KH, Gerelchuluun T, Song S, Kim K, Lee CK. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007. 179:5711–5716.
Article12. Yoo HS, Shin JW, Cho JH, Son CG, Lee YW, Park SY, Cho CK. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin. 2004. 25:657–665.13. Liu J, Yang S, Yang X, Chen Z, Li J. Anticarcinogenic effect and hormonal effect of Cordyceps militaris Link. Zhongguo Zhong Yao Za Zhi. 1997. 22:111–113.14. Zhao-Long W, Xiao-Xia W, Wei-Ying C. Inhibitory effect of Cordyceps sinensis and Cordyceps militaris on human glomerular mesangial cell proliferation induced by native LDL. Cell Biochem Funct. 2000. 18:93–97.15. Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997. 90:1594–1599.
Article16. Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001. 22:141–148.
Article17. Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002. 20:621–667.
Article18. Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976. 143:1283–1288.
Article19. Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995. 5:105–109.
Article20. Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984. 2:283–318.
Article21. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005. 4:281–286.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory Effects of Hypocrellin A on MHC-restricted Antigen Processing
- Lectins Isolated from Mushroom Fomitella fraxinea Enhance MHC-restricted Exogenous Antigen Presentation
- Metformin Suppresses MHC-Restricted Antigen Presentation by Inhibiting Co-Stimulatory Factors and MHC Molecules in APCs
- Evidence for Direct Inhibition of MHC-Restricted Antigen Processing by Dexamethasone
- Inhibition of Major Histocompatibility Complex (MHC)- Restricted Presentation of Exogenous Antigen in Dendritic Cells by Korean Propolis Components