J Bacteriol Virol.
2015 Jun;45(2):138-150. 10.4167/jbv.2015.45.2.138.
CCL5 Inhibits Elevation of Blood Pressure and Expression of Hypertensive Mediators in Developing Hypertension State Spontaneously Hypertensive Rats
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Yeungnam University, Daegu, Korea. heesun@med.yu.ac.kr
- 2Department of Pharmacology, College of Medicine, Yeungnam University, Daegu, Korea.
- 3Department of Physiology, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 2149214
- DOI: http://doi.org/10.4167/jbv.2015.45.2.138
Abstract
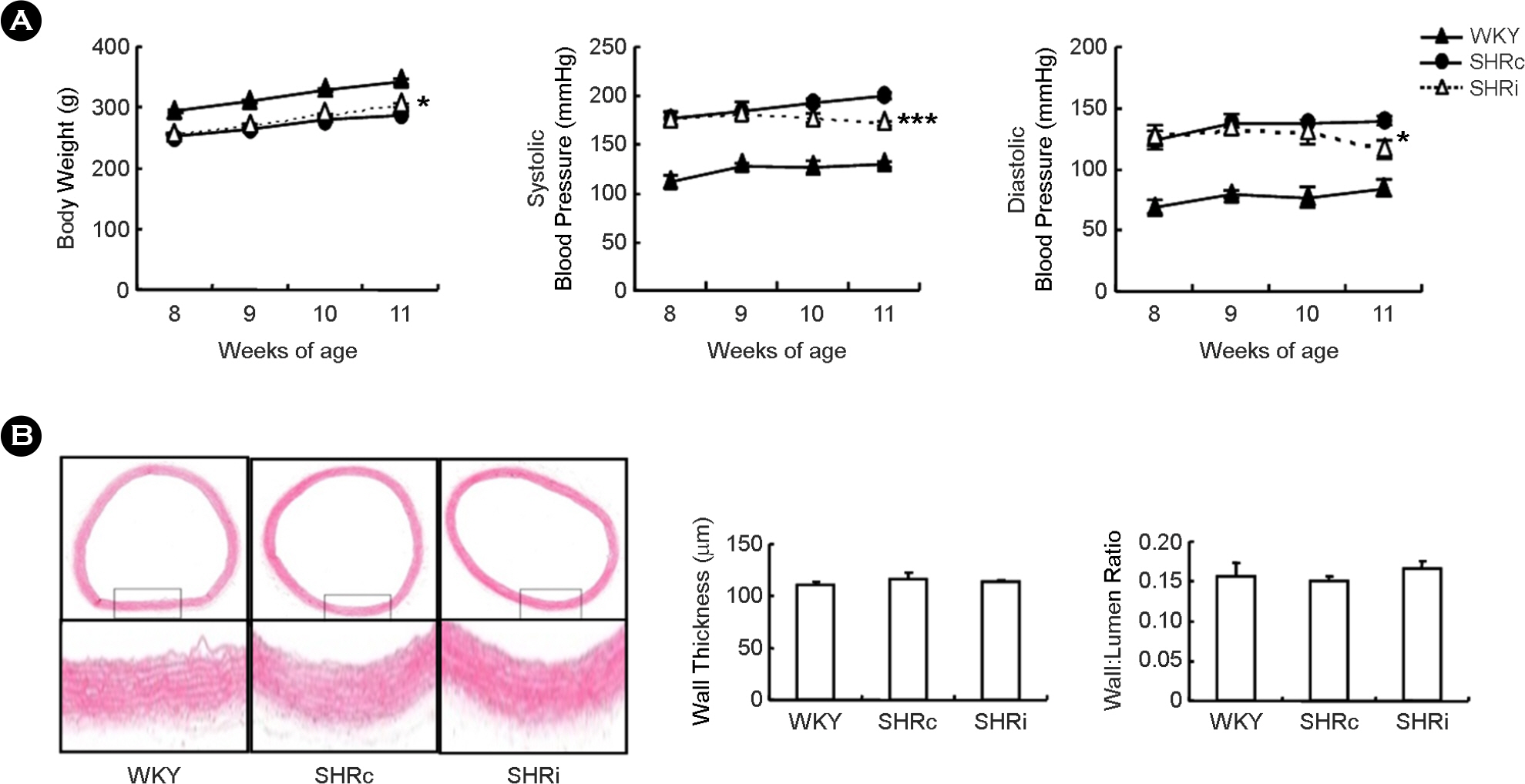
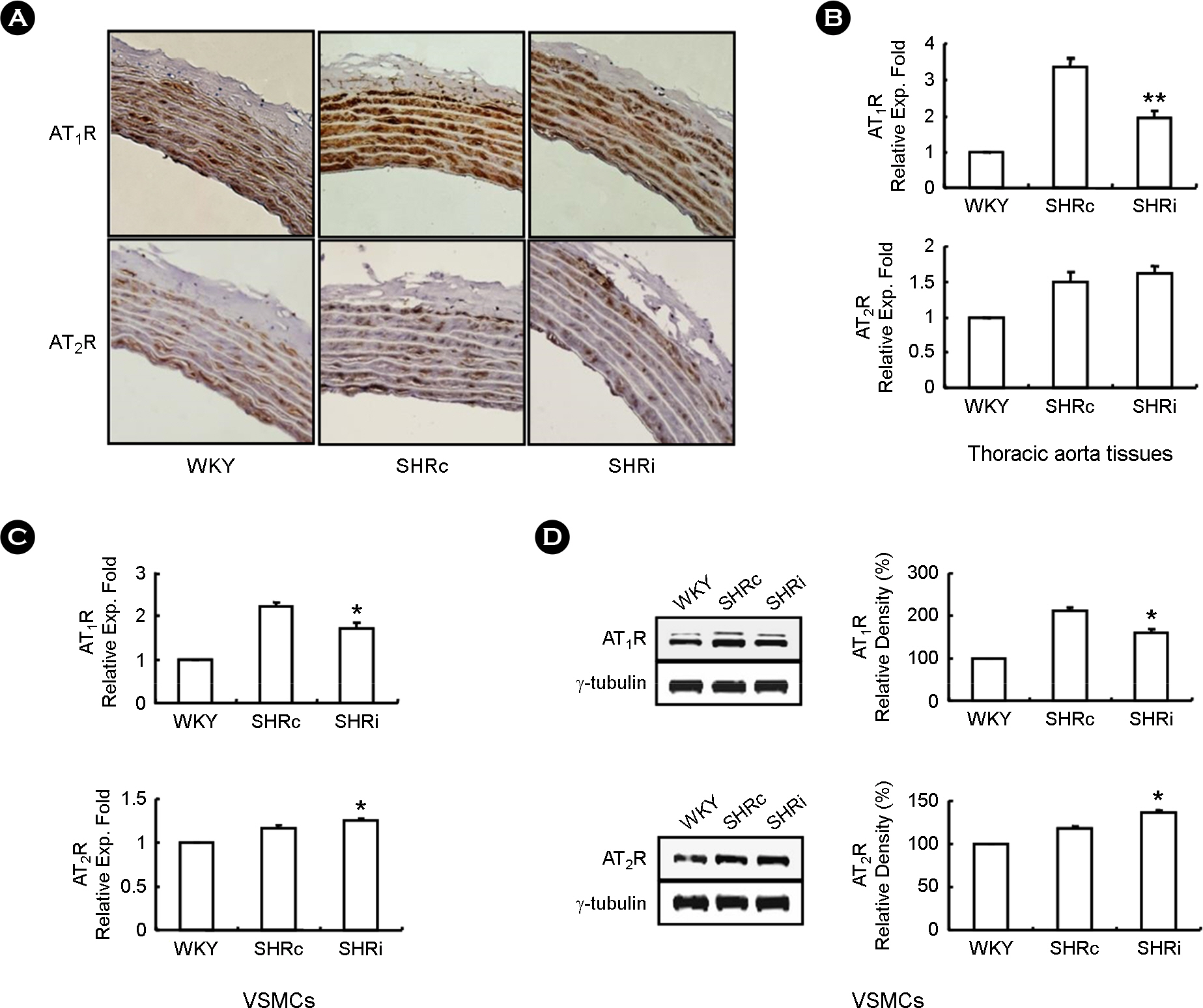
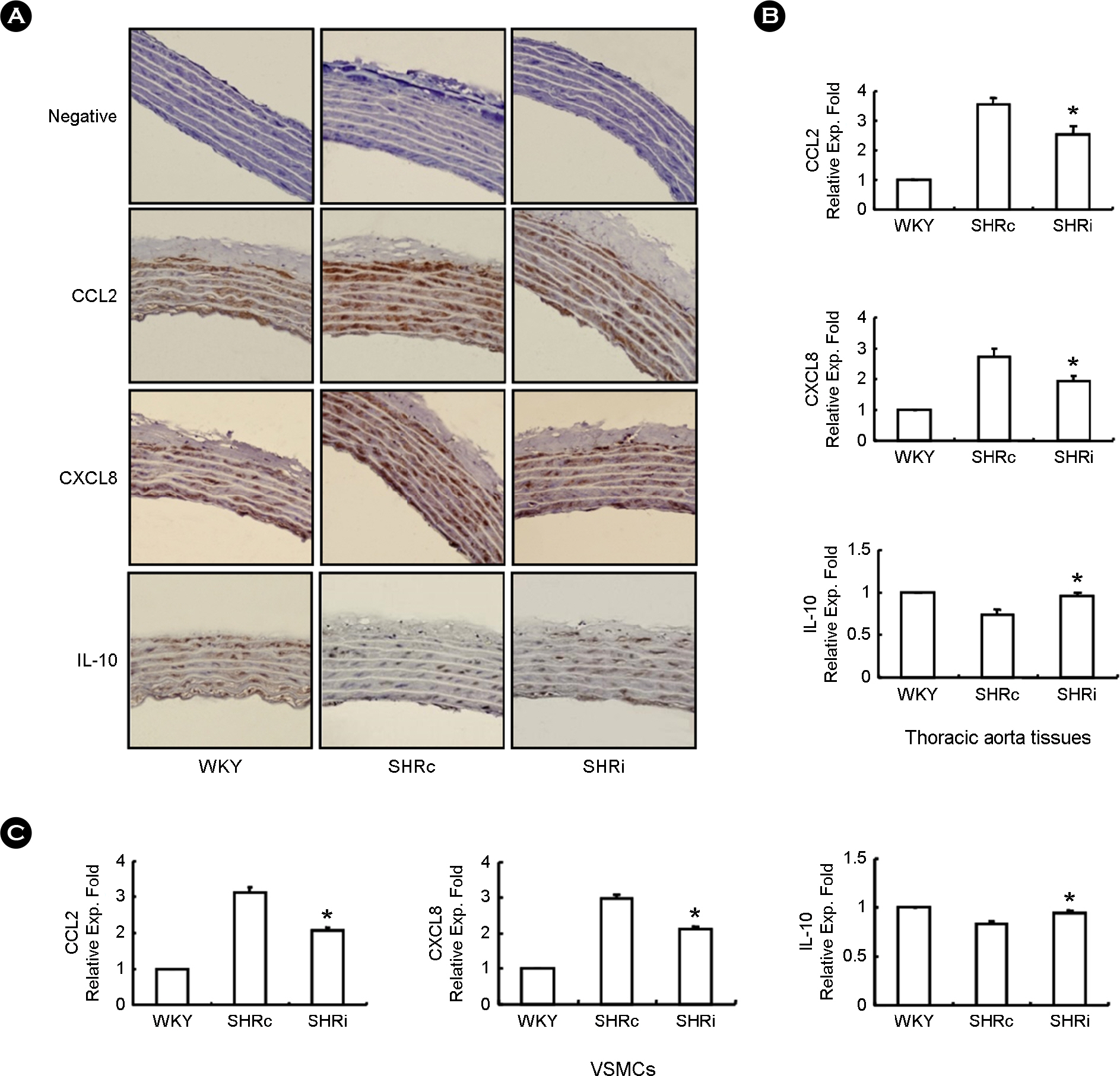
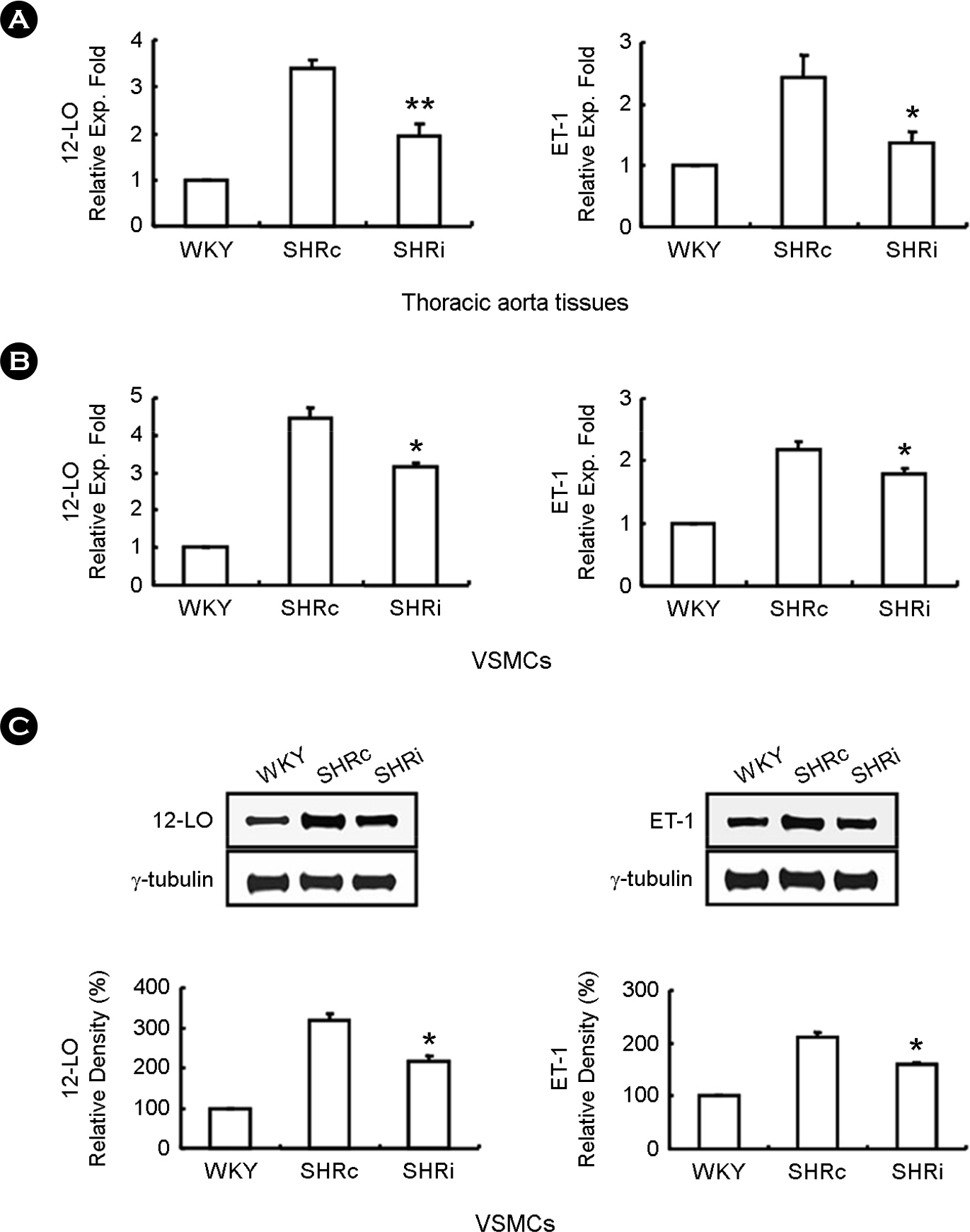
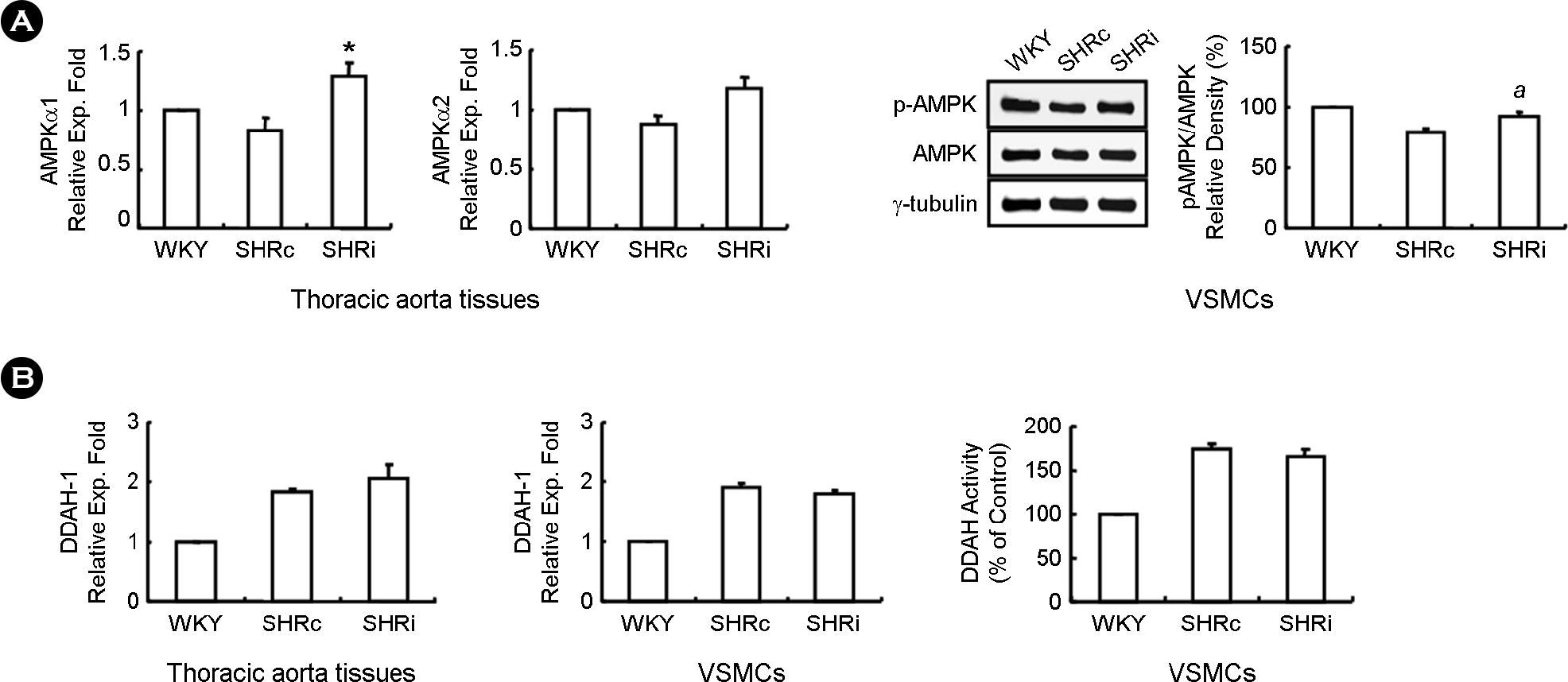
- CCL5, a proinflammatory chemokine, has been shown to attenuate angiotensin (Ang) II-induced expression of hypertensive mediators as well as Ang II-induced inhibition of anti-hypertensive mediator expression in vascular smooth muscle cells (VSMCs) of spontaneously hypertensive rats (SHR). In the present study, functional roles of CCL5 on hypertension were examined in developing hypertension state SHR (DHSHR). DHSHR at an age of 8 weeks were injected CCL5 (1.5 microg/kg) subcutaneously twice a day for 3 weeks (SHRi, n=5). Control groups consisted of normal age-matched saline-treated SHR (SHRc, n=5) and normotensive Wistar-Kyoto rats (WKY, n=5). Effect of CCL5 on blood pressure was measured before treatment, weekly during treatment, and 1 day after the final injection. After injecting for 3 weeks, effects of CCL5 on expression of hypertensive mediators were examined in thoracic aorta tissues and VSMCs. Blood pressure in SHRi was maintained without any elevation during the treatment period, whereas blood pressure in SHRc progressively increased with age. Expression of Ang II subtype I receptor was reduced in SHRi thoracic aorta tissues and VSMCs compared to those in SHRc. In addition, expression levels of hypertensive mediators were significantly reduced in SHRi thoracic aorta tissues and VSMCs compared to those in SHRc. In contrast, AMP-activated protein kinase (AMPK) activity and interleukin-10 (IL-10) expression were elevated in SHRi thoracic aorta tissues and VSMCs compared to levels in SHRc. These results suggest that reduction of hypertensive mediators and elevation of anti-hypertensive mediators by CCL5 treatment promotes maintenance of blood pressure in DHSHR.
MeSH Terms
Figure
Reference
-
1). Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis: oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995; 25:155–61.2). Dhungana S, Sharrack B, Woodroofe N. Cytokines and chemokines in idiopathic intracranial hypertension. Headache. 2009; 49:282–5.
Article3). Capers Q 4th, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, et al. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997; 30:1397–402.
Article4). Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocyte in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004; 94:1203–10.5). Kim HY, Choi JH, Kang YJ, Park SY, Choi HC, Kim HS. Reparixin, an inhibitor of CXCR1 and CXCR2 receptor activation, attenuates blood pressure and hypertension-related mediators expression in spontaneously hypertensive rats. Biol Pharm Bull. 2011; 34:120–7.
Article6). Navratilova Z. Polymorphisms in CCL2 & CCL5 chemokines/chemokine receptors genes and their association with diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006; 150:191–204.7). Dorfmüller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002; 165:534–9.
Article8). Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int. 1998; 53:1608–15.9). Kashiwagi M, Mustutani K, Shinozaki M, Hirakata H. MCP-1 and RANTES are expressed in renal cortex of rats chronically treated with nitric oxide synthase inhibitor. Involvement in macrophage and monocyte recruitment. Nephron. 2002; 92:165–73.10). Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 2006; 177:5077–87.
Article11). Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. 2010; 31:8–16.
Article12). Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, et al. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008; 28:3277–90.
Article13). Kim JH, Kim HS. Downregulation of angiotensin ll-induced 12-lipoxygenase expression and cell proliferation in vascular smooth muscle cells from spontaneously hypertensive rats by CCL5. Korean J Physiol Pharmacol. 2009; 13:385–92.14). Kim HY, Kim JH, Kim HS. Effect of CCL5 on dimethylarginine dimethylaminohydrolase-1 production in vascular smooth muscle cells from spontaneously hypertensive rats. Cytokine. 2013; 64:227–33.
Article15). Kim HY, Cha HJ, Kim HS. CCL5 upregulates activation of AMP-activated protein kinases in vascular smooth muscle cells of spontaneously hypertensive rats. Cytokine. 2014; 67:77–84.
Article16). Kim HY, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008; 31:515–23.
Article17). Kim JH, Kang YJ, Kim HS. IL-8/CXCL8 upregulates 12-lipoxygenase expression in vascular smooth muscle cells from spontaneously hypertensive rats. Immune Netw. 2009; 9:106–13.
Article18). Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J Immunol. 2012; 188:4209–16.
Article19). Ueda S, Kato S, Matsuoka H, Kimoto M, Okuda S, Morimatsu M, et al. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ Res. 2003; 92:226–33.20). Natarajan R, Rosdahl J, Gonzales N, Bai W. Regulation of 12-lipoxygenase by cytokines in vascular smooth muscle cells. Hypertension. 1997; 30:873–9.
Article21). Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens. 1997; 10:371–8.
Article22). Bae JJ, Kim JH, Kim H, Kim HS. Expression of Endothelin-1 by Stimulation with CXCL8 in Mouse Peritoneal Macrophages. J Bacteriol Virol. 2009; 39:205–16.
Article23). Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: Effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens. 2005; 18:81–7.
Article24). Vaziri ND, Ni Z, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998; 31:1248–54.
Article25). Fukuda S, Tsuchikura S, Iida H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 2004; 53:67–72.
Article26). Montecucco F, Braunersreuther V, Lenglet S, Delattre BM, Pelli G, Buatois V, et al. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J. 2012; 33:1964–74.
Article27). Siragy HM. AT(1) and AT(2) receptors in the kidney: role in disease and treatment. Am J Kidney Dis. 2000; 36:4–9.
Article28). Usui M, Egashira K, Tomita H, Koyanagi M, Katoh M, Shimokawa H. Important role of local angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation. 2000; 101:305–10.29). Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F. Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int. 2002; 61:1986–95.30). De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, et al. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens. 1999; 17:1873–7.31). Jin XQ, Fukuda N, Su JZ, Lai YM, Suzuki R, Tahira Y, et al. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension. 2002; 39:1021–7.
Article32). Widdop RE, Vinh A, Henrion D, Jones ES. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008; 35:386–90.33). Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin ll induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998; 83:952–9.34). Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999; 398:718–23.
Article35). Buemi M, Marino D, Floccari F, Ruello A, Nostro L, Aloisi C, et al. Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 2004; 20:19–24.
Article36). Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4 (+)CD25 (+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011; 31:2534–42.37). Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999; 85:e17–24.
Article38). Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol. 2000; 279:H1555–62.
Article39). Kim HY, Kim HS. IL-10 upregulates CCL5 expression in vascular smooth muscle cells from spontaneously hypertensive rats. Cytokine. 2014; 68:40–9.
Article40). Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L367–74.
Article41). Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008; 22:3595–606.
Article42). Reddy MA, Sahar S, Villeneuve LM, Lanting L, Natarajan R. Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009; 29:387–93.
Article43). González-Núñez D, Claria J, Rivera F, Poch E. Increased levels of 12 (S)-HETE in patients with essential hypertension. Hypertension. 2001; 37:334–8.44). Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, Stern N. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol. 1990; 259:H1774–80.
Article45). Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2002; 3:1–15.
Article46). Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989; 86:2863–7.
Article47). Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005; 43:19–29.
Article48). Dumont Y, D'Amours M, Lebel M, Larivière R. Blood pressure-independent effect of angiotensin AT1 receptor blockade on renal endothelin-1 production in hypertensive uremic rats. J Hypertens. 2001; 19:1479–87.
Article49). Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens. 2012; 30:725–33.
Article50). Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol. 2013; 304:H1159–65.
Article51). Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, et al. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun. 2012; 418:559–64.
Article52). Teng RJ, Du J, Afolayan AJ, Eis A, Shi Y, Konduri GG. AMP kinase activation improves angiogenesis in pulmonary artery endothelial cells with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013; 304:L29–42.
Article53). Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, et al. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004; 110:444–51.
Article54). Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007; 293:H3227–45.
Article55). Wadham C, Mangoni AA. Dimethylarginine dimethylaminohydrolase regulation: a novel therapeutic target in cardiovascular disease. Expert Opin Drug Metab Toxicol. 2009; 5:303–19.
Article56). Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003; 23:1455–9.
Article57). Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999; 343:209–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- Pressor Responses to Intracerebroventricular Infusion of Hypertonic NaCl in Renal Hypertensive Rats
- Hypertensive Encephalopathy in a 10-year-old Boy with Ureteral Stone
- Expression of Class I and Class II a/b Histone Deacetylase is Dysregulated in Hypertensive Animal Models
- Altered renal expression of nitric oxide synthase isozymes in spontaneously hypertensive rats