Cancer Res Treat.
2004 Apr;36(2):115-120.
Oxaliplatin with Biweekly, Low Dose Leucovorin and Bolus and Continuous Infusion 5-fluorouracil (Modified FOLFOX 4) as First-line Therapy for Patients with Metastatic Colorectal Cancer
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. kimhj@mail.donga.ac.kr
Abstract
- PURPOSE
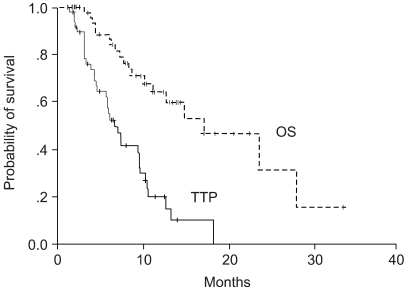
To determine the activity and toxicities of low dose leucovorin (LV) plus fluorouracil (5-FU) regimen, combined with oxaliplatin every two weeks (modified FOLFOX 4), as a first-line therapy for patients with metastatic colorectal cancer. MATERIALS AND METHODS: Between March 2001 and August 2003, fifty-five patients were enrolled in this study. Patients were treated with oxaliplatin 85 mg/m2 as a 2-hour infusion at days 1 plus LV 20 mg/m2 over 10 minutes, followed by 5-FU bolusa 400 mg/m2 bolus and 22 hour continuous infusion of 600 mg/m2 5-FU at day 1~2. This treatment was repeated in 2 week intervals. RESULTS: The objective response rate was 40% on an intent-to-treatment analysis. Three patients (6%) demonstrated a complete response and nineteen patients (38%) showeda partial response. Sixteen patients (32%) showed a stable disease and eleven patients (22%) progressed during the course of the treatment. The median time to progression and overall survival time wereas 6.6 months (95% CI: 4.98~8.02 months) and the median overall survival time was 17.0 months (95% CI: 9.15~24.85 months) from the start of the chemotherapy, respectively. A total of 275 cycles were analyzed for toxicity. Major hematologic toxicities included grade 1~2 anemia (23.5%), neutropenia (25.3%) and thrombocytopenia (10.6%). There were only 2 cycles of neutropenic fever. The most common non- hematologic toxicities were grade 1~2 nausea/vomiting (10.9%), diarrhea (9.1%) and grade 1 neuropathy (18.0%). There was no treatment related death. CONCLUSION: The modified folfox 4 regimen is safe and effective regimen as a first-line therapy in advanced colorectal cancer patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Shin HR, Ahn YO, Bae JM, Shin MH, Lee DH, Lee CW, Ohrr H, Ahn DH, Ferlay J, Parkin DM, Oh DK, Park JG, Bae JM, Won YJ, Jung KW, Park JG. Cancer incidence in Korea. Cancer Res Treat. 2002; 34:405–408.
Article2. American Cancer Society: Cancer Facts and Figures-2000. 2000. Atlanta, GA: American Cancer Society.3. Jonker DJ, Maroun JA, Kocha W. Survival benefit of chemotherapy in metastatic colorectal cancer: A meta-analysis of randomised controlled trials. Br J Cancer. 2000; 82:1789–1794. PMID: 10839292.4. Water J, Cunningham D. The changing face of chemotherapy in colorectal cancer. Br J Cancer. 2001; 84:1–7.
Article5. Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast, and ovarian cancers. Anticancer Drugs. 1997; 8:876–885. PMID: 9402315.
Article6. Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, Itzhaki M, Metzger G, N'Daw D, Vignoud J, Abad A, Francois E, Gamelin E, Marty M, Sastre J, Seitz JF, Ychou M. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996; 7:95–98. PMID: 9081400.
Article7. Becouam Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL, Duffour J, Fandi A, Dupont-Andre G, Rougier P. A phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol. 1998; 16:2739–2744. PMID: 9704726.8. Levi F, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L. Chronomodulated versus fixed infusion rate delivery of ambulatory chemotherapy with oxaliplatin, 5-fluorouracil and folinic acid in patients with colorectal cancer metastases: A randomized multi-institutional trial. J Natl Cancer Inst. 1994; 86:1608–1617. PMID: 7932825.9. de Gramont A, Vignoud J, Tournigand C, Louvet C, Andre T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997; 33:214–219. PMID: 9135491.
Article10. Bae YZ, Jung JH, Moon CH, Kim SH, Kwon HC, Kim JS, Kim HJ. A phase II study of oxaliplatin combined with 5-fluorouracil and leucovorin (Mayo Clinic regimen) in 5-fluorouracil refractory colorectal cancer. Cancer Res Treat. 2002; 34:218–222.
Article11. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000; 355:1041–1047. PMID: 10744089.
Article12. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947. PMID: 10944126.
Article13. Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000; 18:136–147. PMID: 10623704.
Article14. Goldberg RM, Morton RF, Sargent DJ, Fuchs C, Ramanathan RK, Williamson SK, Findly BP. N9741: Oxaliplatin (oxal) or CPT-11+5-fluorouracil (5FU)/leucovorin (LV) or oxal+CPT-11 in advanced colorectal cancer(CRC). Updated efficacy and quality of life (QOL) data from an intergroup study. Proc Am Soc Clin Oncol. 2003; 22:252. (abstr 1009).15. Ramanathan RK, Clark JW, Kemeny NE, Lenz H, Gococo KO, Haller DG, Mitchell EP, Kardinal CG. Safety and Toxicity Analysis of Oxaliplatin Combined With Fluorouracil or as a Single Agent in Patients With Previously Treated Advanced Colorectal Cancer. J Clin Oncol. 2003; 21:2904–2911. PMID: 12885808.
Article16. Borner MM, Dietrich D, Stupp R, Morant R, Honegger H, Wernli M, Herrmann R, Pestalozzi BC, Saletti P, Hanselmann S, Muller S, Brauchli P, Castiglione-Gertsch M, Goldhirsch A, Roth AD. Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorectal cancer. J Clin Oncol. 2002; 20:1759–1766. PMID: 11919232.
Article17. Maindrault-Goebel F, de Gramont A, Louvet C, Andre T, Carola E, Mabro M, Artru P, Gilles V, Lotz JP, Izrael V, Krulik M. Oncology Multidisciplinary Research Group (GERCOR). High-dose intensity oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as secondline therapy for metastatic colorectal cancer (FOLFOX 7). Eur J Cancer. 2001; 37:1000–1005. PMID: 11334725.18. Sastre J, Butts C, Cassidy J, Conroy T, DeBraud F, Figer A, Schoeffski P, Tabernero J, Twelves C, Cutsem EV. Capecitabine-oxaliplatin combination (XELOX), an effective first-line therapy for patietnspatients with metastatic colorectal cancer: Survival updates of an international phase II trial. Ann Oncol. 2002; 13:80. (abstr 288).19. Ducreaux M, Bouche O, Pignon JP, Raoul JL, Mousseau M, Deguiral P, Berger C, Cassan P, Leduc B, Bedenne L. Randomized trial comparing three different schedules of infusional 5FU and raltitrexed alone in first line metastatic colorectal cancer. Final results of the Federation Francophone de Cancerologie Digestive 9601 trial. Ann Oncol. 2002; 13:71. (abstr 259).20. Cavaletti G, Tredici G, Petruccioli MG, Donde E, Tredici P, Marmiroli P, Minoia C, Ronchi A, Bayssas M, Etienne GG. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer. 2001; 37:2457–2463. PMID: 11720843.
Article21. Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: Current management and development of preventive measures. Semin Oncol. 2002; 29:21–33. PMID: 12422305.
Article22. Cascinu S, Catalano V, Cordella L, Labianca R, Giordani P, Baldelli AM, Beretta GD, Ubiali E, Catalano G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002; 20:3478–3483. PMID: 12177109.
Article23. Falcone A, Masi G, Allegrini G, Danesi R, Pfanner E, Brunetti IM, Paolo AD, Cupini S, Tacca MD, Conte P. Biweekly Chemotherapy With Oxaliplatin, Irinotecan, Infusional Fluorouracil, and Leucovorin: A Pilot Study in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2002; 20:4006–4014. PMID: 12351598.
Article24. Hurwitz H, Fehrenbacher L, Cartwright T, Hainsworth J, Heim W, Berlin J, Griffing S, Novotny W, Holmgren E, Kabbinavar F. Results of a phase III trial of bevacizumab in combination with bolus irinotecan, 5-fluourouracil, leucovorin as first-line therapy in subjects with metastatic CRC. Proc Am Soc Clin Oncol. 2003; 22:(abstr 3646).25. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Van E. Cetuximab (C225) alone or in combination with irinotecan in patients with epidermal growth factor receptor-positive, irinotecan-refractory metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2003; 22:252. (abstr 1012).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oxaliplatin with Biweekly Low Dose Leucovorin and Bolus and Continuous Infusion of 5-fluorouracil (Modified FOLFOX 4) as a Salvage Therapy for Patients with Advanced Gastric Cancer
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- Combination Chemotherapy of Oxaliplatin, 5-Fluorouracil and Low Dose Leucovorin in Patients with Advanced Colorectal Cancer
- Comparison between Responder and Non- responder of Oxaliplatin Chemotherapy for Metastatic Colorectal Cancer
- Differences of Response Rates according to Metastatic Sites after Oxaliplatin, 5- Fluorouracil, and Leucovorin Combination Chemotherapy (FOLFOX 3) in Advanced Colorectal Cancer