Korean J Physiol Pharmacol.
2012 Apr;16(2):139-144. 10.4196/kjpp.2012.16.2.139.
TRPC-Mediated Current Is Not Involved in Endocannabinoid-Induced Short-Term Depression in Cerebellum
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea. sangjkim@snu.ac.kr
- 2Department of Brain and Cognitive Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 2071760
- DOI: http://doi.org/10.4196/kjpp.2012.16.2.139
Abstract
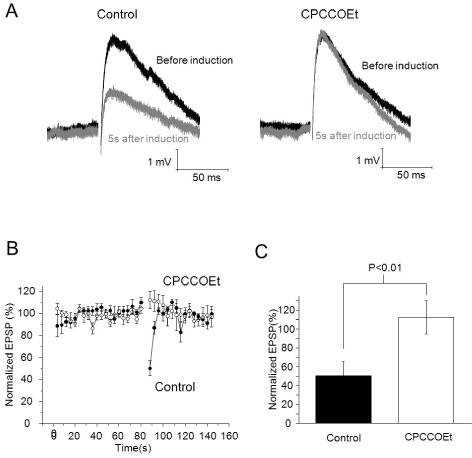
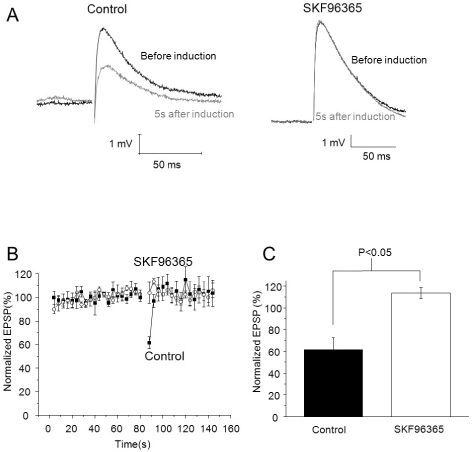
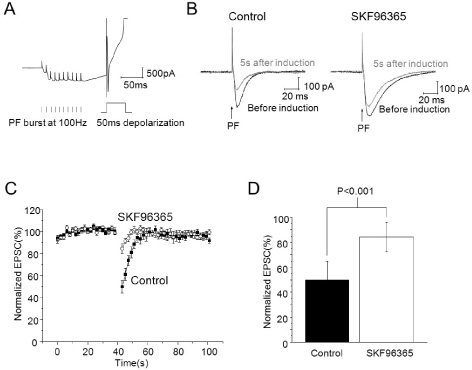
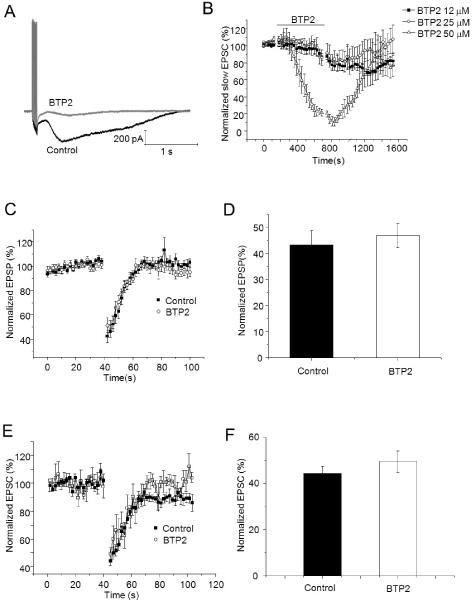
- It has been reported that activation of metabotropic glutamate receptor 1 (mGluR1) can mediate endocannabinoid-induced short-term depression of synaptic transmission in cerebellar parallel fiber (PF)-Purkinje cell (PC) synapse. mGluR1 has signaling pathways involved in intracellular calcium increase which may contribute to endocannabinoid release. Two major mGluR1-evoked calcium signaling pathways are known: (1) slow-kinetic inward current carried by transient receptor potential canonical (TRPC) channel which is permeable to Ca2+; (2) IP3-induced calcium release from intracellular calcium store. However, it is unclear how much each calcium source contributes to endocannabinoid signaling. Here, we investigated whether calcium influx through mGluR1-evoked TRPC channel contributes to endocannabinoid signaling in cerebellar Purkinje cells. At first, we applied SKF96365 to inhibit TRPC, which blocked endocannabinoid-induced short-term depression completely. However, an alternative TRP channel inhibitor, BTP2 did not affect endocannabinoid-induced short-term depression although it blocked mGluR1-evoked TRPC currents. Endocannabinoid signaling occurred normally even though the TRPC current was mostly blocked by BTP2. Our data imply that TRPC current does not play an important role in endocannabinoid signaling. We also suggest precaution in applying SKF96365 to inhibit TRP channels and propose BTP2 as an alternative TRPC inhibitor.
MeSH Terms
Figure
Reference
-
1. Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997. 37:205–237.2. Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994. 13:1031–1037.3. Lee J, Kim I, Oh SR, Ko SJ, Lim MK, Kim DG, Kim CH. Regulation of DREAM expression by group I mGluR. Korean J Physiol Pharmacol. 2011. 15:95–100.4. Mockett BG, Guévremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci. 2011. 31:7380–7391.5. Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998. 396:757–760.6. Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998. 396:753–756.7. Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998. 80:520–528.8. Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003. 426:285–291.9. Chuang SC, Bianchi R, Wong RK. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000. 83:2844–2853.10. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990. 346:561–564.11. Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992. 106:231–232.12. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990. 87:1932–1936.13. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992. 258:1946–1949.14. Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997. 388:773–778.15. Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002. 22:1690–1697.16. Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci. 2005. 25:6826–6835.17. Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008. 54:213–218.18. Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994. 13:1447–1455.19. Wagner JJ, Alger BE. Increased neuronal excitability during depolarization-induced suppression of inhibition in rat hippocampus. J Physiol. 1996. 495:107–112.20. Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002. 15:953–961.21. Kim JH, PE , Chang DJ, Choi SW. Modulation of amygdala synaptic transmission by metabotropic glutamate receptors. Korean J Physiol Pharmacol. 2003. 7:303–306.22. Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005. 45:419–431.23. Hartmann J, Konnerth A. Mechanisms of metabotropic glutamate receptor-mediated synaptic signaling in cerebellar Purkinje cells. Acta Physiol (Oxf). 2008. [Epub ahead of print].24. Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990. 271:515–522.25. Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010. 160:1464–1475.26. He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005. 280:10997–11006.27. Harper JL, Daly JW. Effect of calmidazolium analogs on calcium influx in HL-60 cells. Biochem Pharmacol. 2000. 60:317–324.28. Ciardo A, Meldolesi J. Multiple actions of SC 38249: the blocker of both voltage-operated and second messenger-operated Ca2+ channels also inhibits Ca2+ extrusion. Eur J Pharmacol. 1990. 188:417–421.29. Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca2+-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992. 267:11789–11793.30. Schwarz G, Droogmans G, Nilius B. Multiple effects of SK&F 96365 on ionic currents and intracellular calcium in human endothelial cells. Cell Calcium. 1994. 15:45–54.31. Capdevila J, Gil L, Orellana M, Marnett LJ, Mason JI, Yadagiri P, Falck JR. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch Biochem Biophys. 1988. 261:257–263.32. Rodrigues AD, Gibson GG, Ioannides C, Parke DV. Interactions of imidazole antifungal agents with purified cytochrome P-450 proteins. Biochem Pharmacol. 1987. 36:4277–4281.33. Mason MJ, Mayer B, Hymel LJ. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am J Physiol. 1993. 264:C654–C662.34. Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, Yamada T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J Immunol. 2003. 170:4441–4449.35. Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004. 279:12427–12437.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Study on the Distribution of Canonical Transient Receptor Potential Channels in Rat Cerebellum
- Metabotropic glutamate receptor dependent long-term depression in the cortex
- Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s
- Modulation of Amygdala Synaptic Transmission by Metabotropic Glutamate Receptors
- Periodic Alternating Nystagmus in Patients with Cerebellar Abscess