Korean J Physiol Pharmacol.
2017 Jan;21(1):133-140. 10.4196/kjpp.2017.21.1.133.
Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. insuk@snu.ac.kr
- 2Department of Physiology, College of Medicine, Gachon University, Incheon 21936, Korea.
- KMID: 2371077
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.133
Abstract
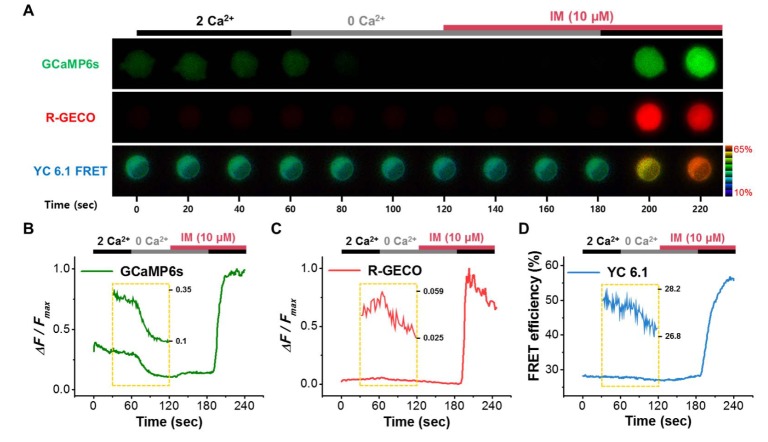
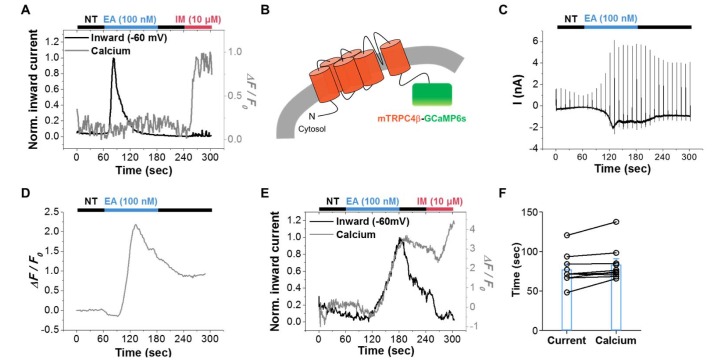
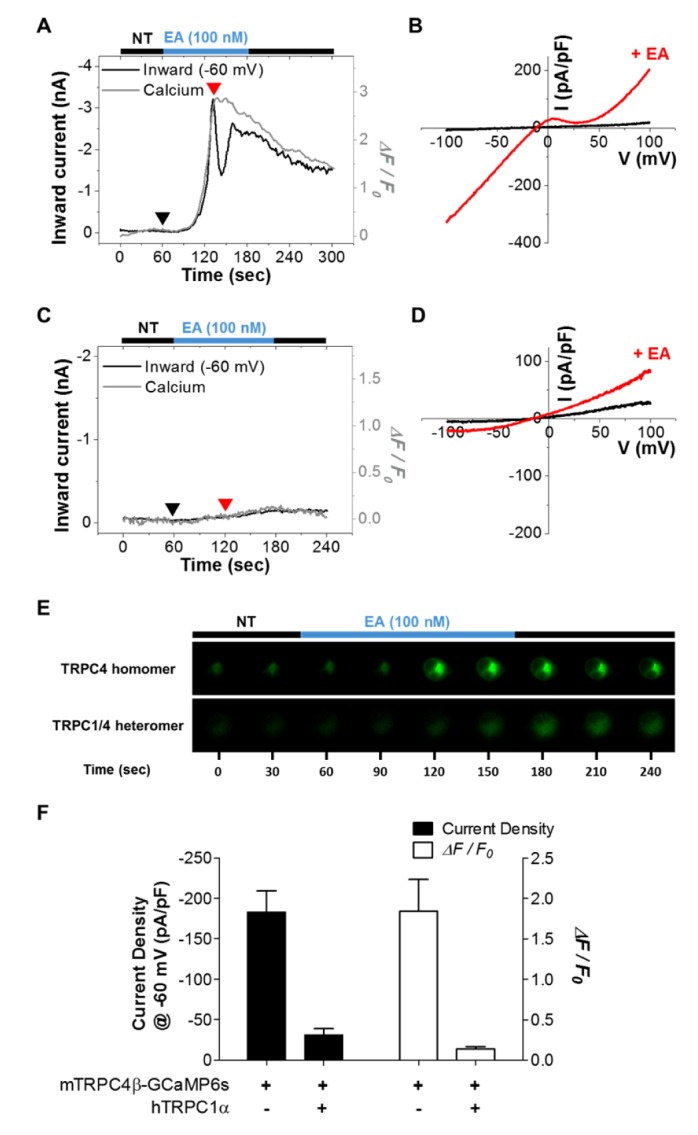
- Conflicting evidence has been obtained regarding whether transient receptor potential cation channels (TRPC) are store-operated channels (SOCs) or receptor-operated channels (ROCs). Moreover, the Ca/Na permeability ratio differs depending on whether the current-voltage (I-V) curve has a doubly rectifying shape or inward rectifying shape. To investigate the calcium permeability of TRPC4 channels, we attached GCaMP6s to TRPC4 and simultaneously measured the current and calcium signals. A TRPC4 specific activator, (-)-englerin A, induced both current and calcium fluorescence with the similar time course. Muscarinic receptor stimulator, carbachol, also induced both current and calcium fluorescence with the similar time course. By forming heteromers with TRPC4, TRPC1 significantly reduced the inward current with outward rectifying I-V curve, which also caused the decrease of calcium fluorescence intensity. These results suggest that GCaMP6s attached to TRPC4 can detect slight calcium changes near TRPC4 channels. Consequently, TRPC4-GCaMP6s can be a useful tool for testing the calcium permeability of TRPC4 channels.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Englerin A-sensing charged residues for transient receptor potential canonical 5 channel activation
SeungJoo Jeong, Juyeon Ko, Minji Kim, Ki Chul Park, Eunice Yon June Park, Jinsung Kim, Youngjoo Baik, Jinhong Wie, Art E. Cho, Ju-hong Jeon, Insuk So
Korean J Physiol Pharmacol. 2019;23(3):191-201. doi: 10.4196/kjpp.2019.23.3.191.Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels
Juyeon Ko, Jongyun Myeong, Misun Kwak, Ju-Hong Jeon, Insuk So
Korean J Physiol Pharmacol. 2019;23(5):357-366. doi: 10.4196/kjpp.2019.23.5.357.
Reference
-
1. Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989; 2:1313–1323. PMID: 2516726.
Article2. Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005; 451:1–10. PMID: 16012814.
Article3. Putney JW. Physiological mechanisms of TRPC activation. Pflugers Arch. 2005; 451:29–34. PMID: 16133266.
Article4. Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001; 15:1727–1738. PMID: 11481220.5. Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001; 3:121–127. PMID: 11175743.6. Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG, Kang DE, Kim HJ, So I. Activation of TRPC4b by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium. 2013; 54:307–319. PMID: 24011658.7. Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996; 15:6166–6171. PMID: 8947038.
Article8. Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000; 275:17517–17526. PMID: 10837492.
Article9. Kim H, Kim J, Jeon JP, Myeong J, Wie J, Hong C, Kim HJ, Jeon JH, So I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin). 2012; 6:333–343. PMID: 22878724.
Article10. Kim SJ, Koh EM, Kang TM, Kim YC, So I, Isenberg G, Kim KW. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. J Physiol. 1998; 513:749–760. PMID: 9824715.11. Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005; 280:30788–30796. PMID: 15987684.12. Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009; 133:525–546. PMID: 19398778.
Article13. Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DS, Yang DK, So I, Kim KW. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol. 2006; 290:C1031–C1040. PMID: 16306123.
Article14. Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y. Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol. 2006; 570:219–235. PMID: 16284075.15. Thakur DP, Tian JB, Jeon J, Xiong J, Huang Y, Flockerzi V, Zhu MX. Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A. 2016; 113:1092–1097. PMID: 26755577.
Article16. Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012; 3:731. PMID: 22415822.
Article17. Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001; 31:973–985. PMID: 11580897.18. Epe B, Steinhäuser KG, Woolley P. Theory of measurement of Förster-type energy transfer in macromolecules. Proc Natl Acad Sci U S A. 1983; 80:2579–2583. PMID: 16593305.19. Patterson G, Day RN, Piston D. Fluorescent protein spectra. J Cell Sci. 2001; 114:837–838. PMID: 11181166.
Article20. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791. PMID: 25707820.21. Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, Yeola S, Lao J, McPate M, Verkuyl JM, Marsh G, Sarber J, Amaral A, Bailey S, Lubicka D, Pham H, Miranda N, Ding J, Tang HM, Ju H, Tranter P, Ji N, Krastel P, Jain RK, Schumacher AM, Loureiro JJ, George E, Berellini G, Ross NT, Bushell SM, Erdemli G, Solomon JM. Englerin a agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One. 2015; 10:e0127498. PMID: 26098886.
Article22. Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004; 287:C357–C364. PMID: 15044151.23. Hong C, Kwak M, Myeong J, Ha K, Wie J, Jeon JH, So I. Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. 2015; 467:703–712. PMID: 24859801.
Article24. Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, Kim SY, Jeon JH, Kim HJ, So I. Isoform- and receptor-specific channel property of canonical transient receptor potential (TRPC)1/4 channels. Pflugers Arch. 2014; 466:491–504. PMID: 23948741.
Article25. Myeong J, Kwak M, Hong C, Jeon JH, So I. Identification of a membrane-targeting domain of the transient receptor potential canonical (TRPC)4 channel unrelated to its formation of a tetrameric structure. J Biol Chem. 2014; 289:34990–35002. PMID: 25349210.
Article26. Myeong J, Kwak M, Jeon JP, Hong C, Jeon JH, So I. Close spatio-association of the transient receptor potential canonical 4 (TRPC4) channel with Gαi in TRPC4 activation process. Am J Physiol Cell Physiol. 2015; 308:C879–C889. PMID: 25788576.
Article27. Myeong J, Ko J, Hong C, Yang D, Lee KP, Jeon JH, So I. The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochem Biophys Res Commun. 2016; 474:476–481. PMID: 27131740.
Article28. Storch U, Forst AL, Philipp M, Gudermann T, Mederos y Schnitzler M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012; 287:3530–3540. PMID: 22157757.
Article29. Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013; 499:295–300. PMID: 23868258.
Article30. Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005; 97:1164–1172. PMID: 16254212.31. Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, Uhlig S, Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res. 2012; 110:1435–1444. PMID: 22534489.
Article32. Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalié A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009; 284:34423–34432. PMID: 19815560.33. Lee KP, Jun JY, Chang IY, Suh SH, So I, Kim KW. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cells. 2005; 20:435–441. PMID: 16404161.34. Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009; 137:1415–1424. PMID: 19549525.
Article35. Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007; 9:636–645. PMID: 17486119.
Article36. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008; 105:2895–2900. PMID: 18287061.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- Immunohistochemical Study on the Distribution of Canonical Transient Receptor Potential Channels in Rat Cerebellum
- Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4
- Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels
- Transient Receptor Potential Canonical 4 and 5 Channel Antagonist ML204 Depolarized Pacemaker Potentials of Interstitial Cells of Cajal