Korean J Physiol Pharmacol.
2009 Apr;13(2):115-121. 10.4196/kjpp.2009.13.2.115.
Antitumor Effects of Camptothecin Combined with Conventional Anticancer Drugs on the Cervical and Uterine Squamous Cell Carcinoma Cell Line SiHa
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. leecs@cau.ac.kr
- 2Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 3Research Institute for Translational System Biomics, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2071660
- DOI: http://doi.org/10.4196/kjpp.2009.13.2.115
Abstract
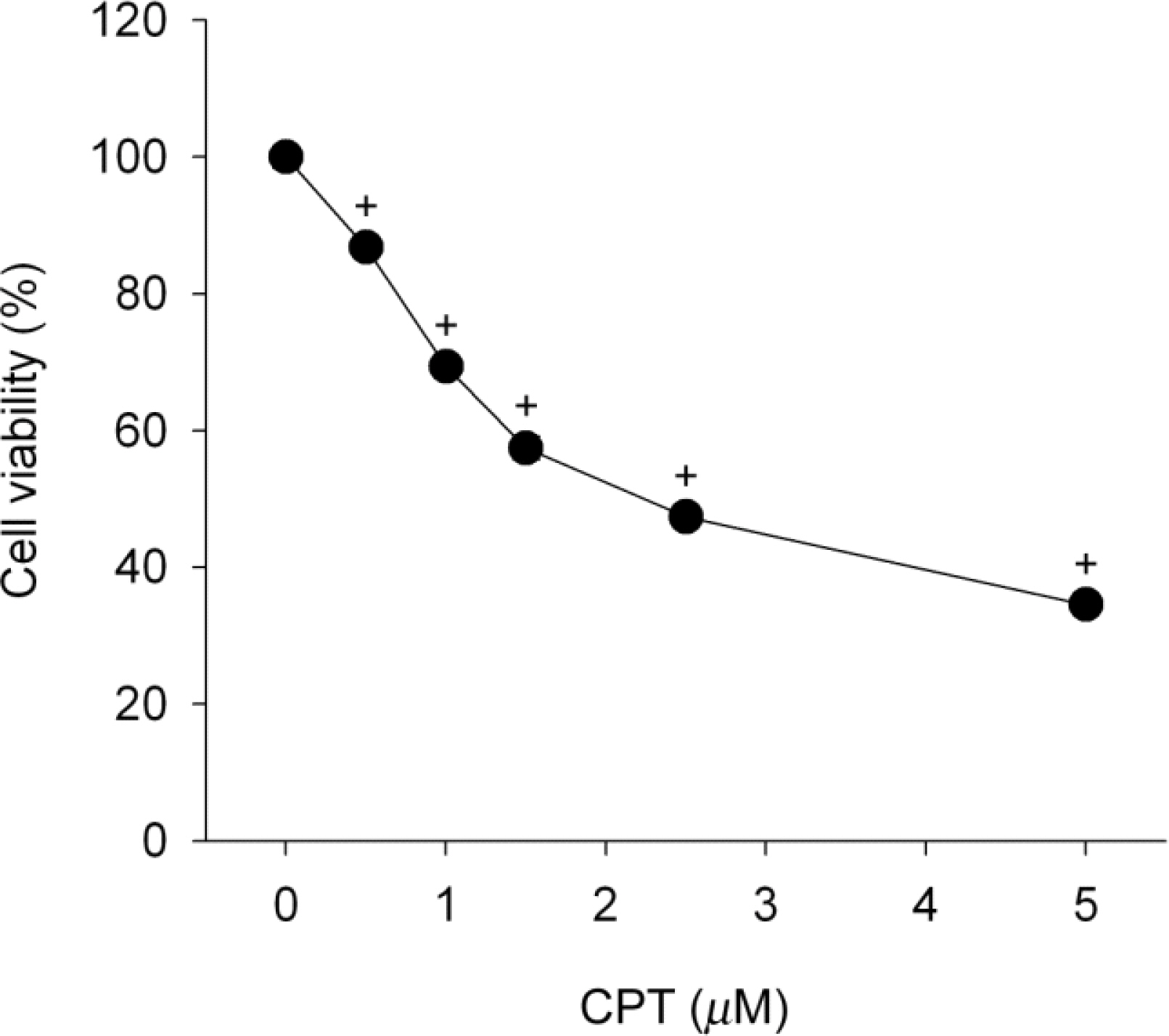
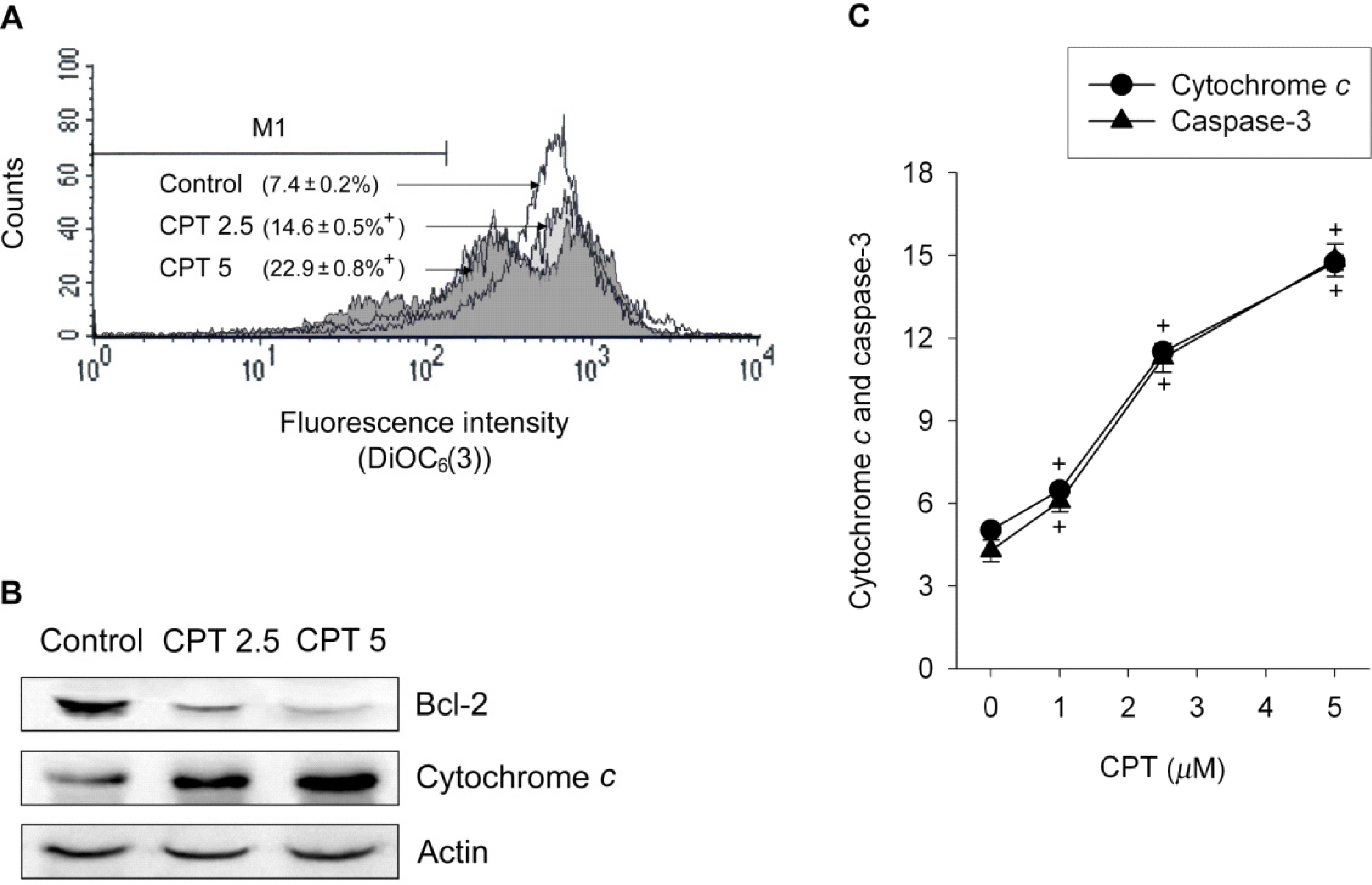
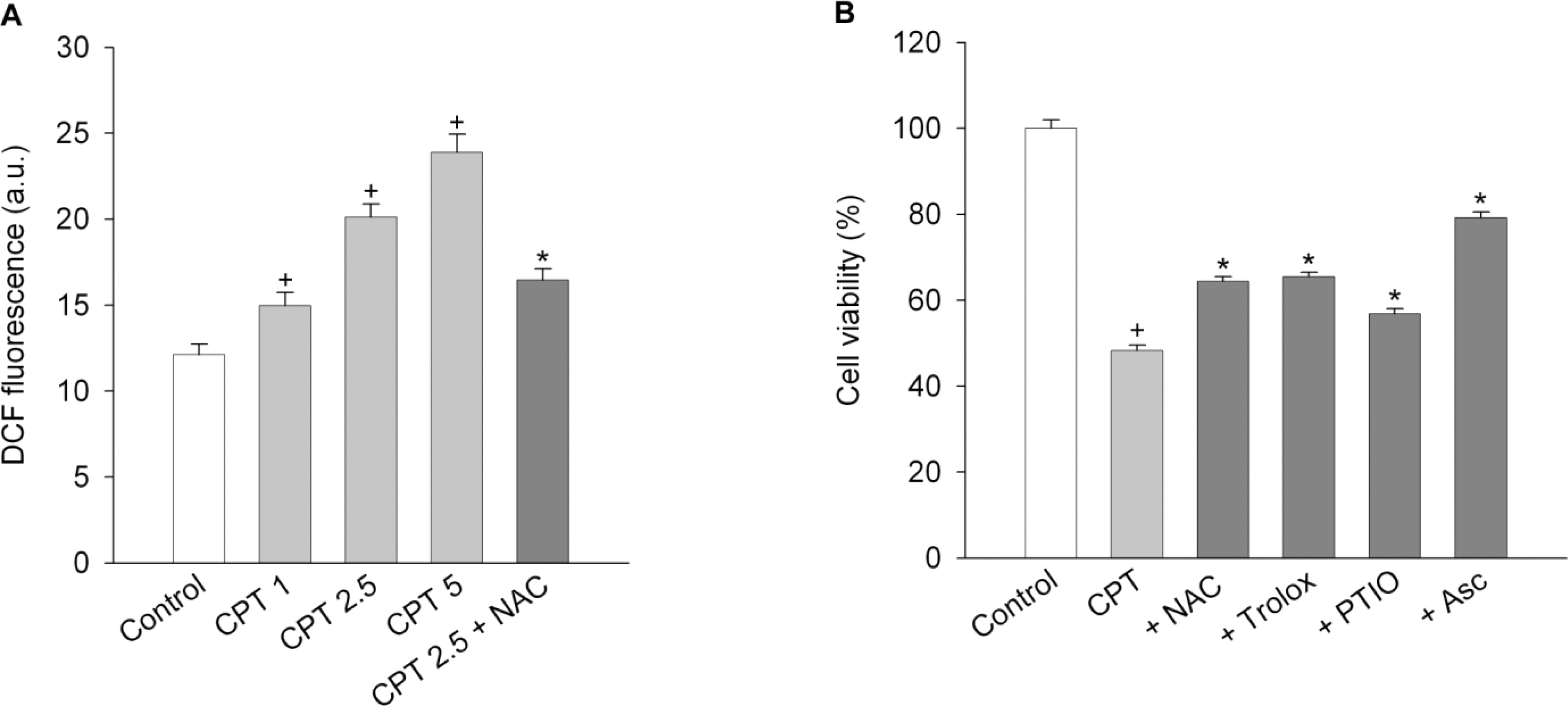
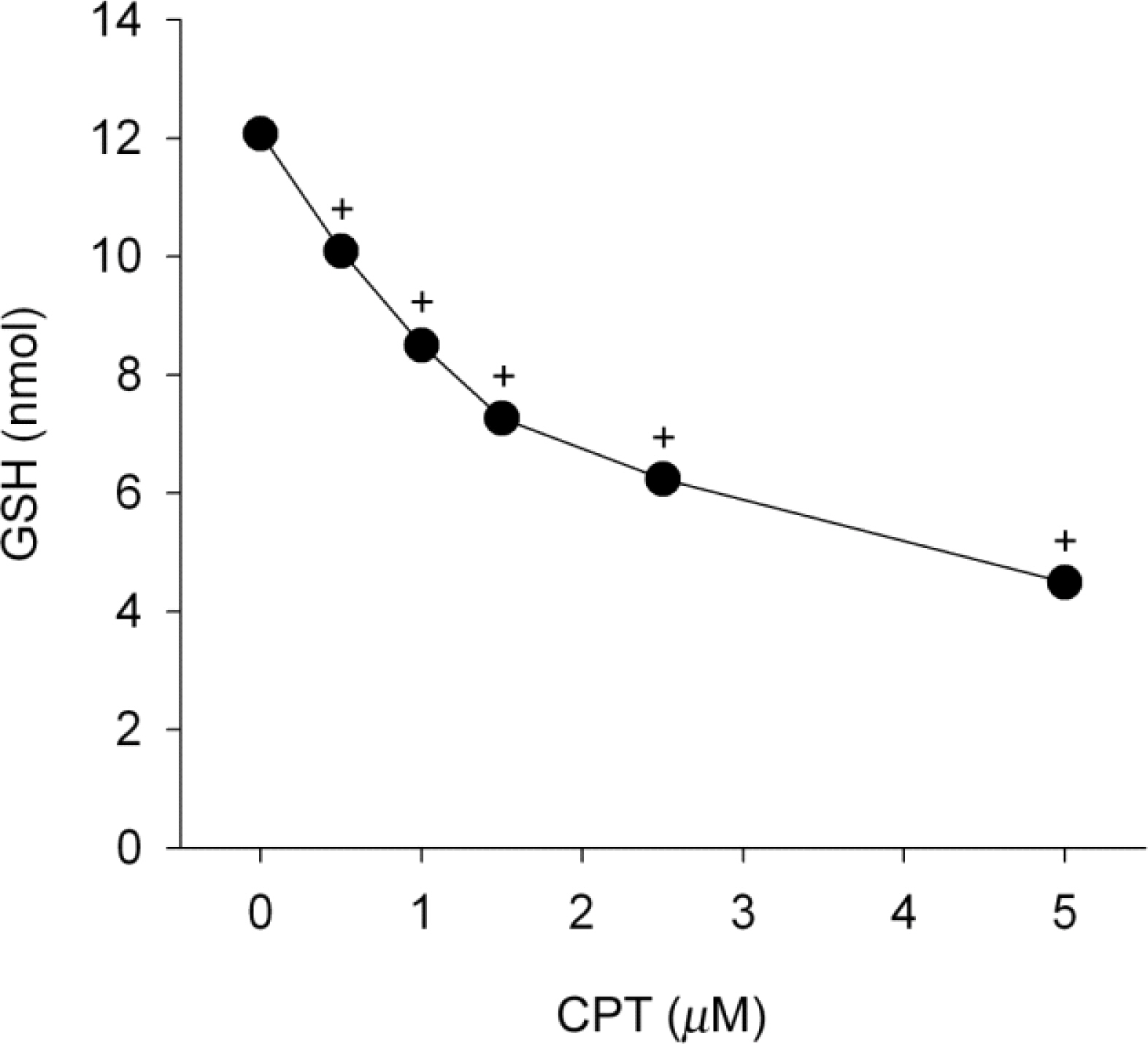
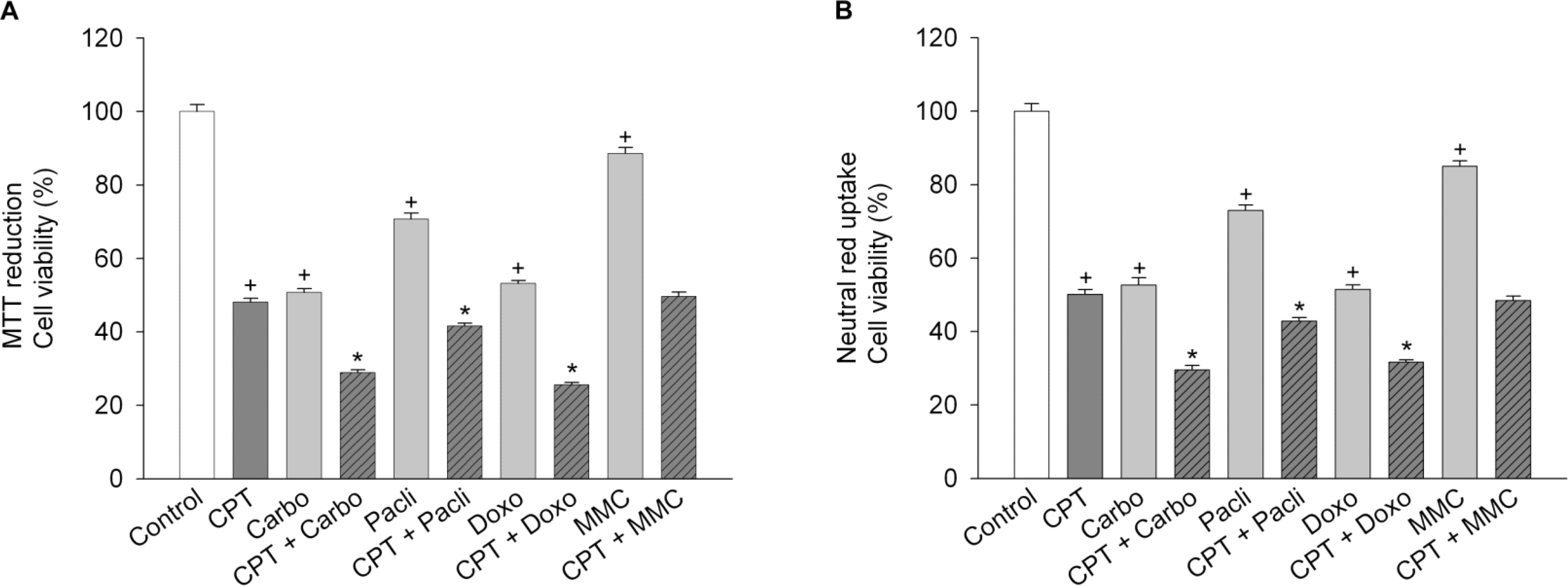
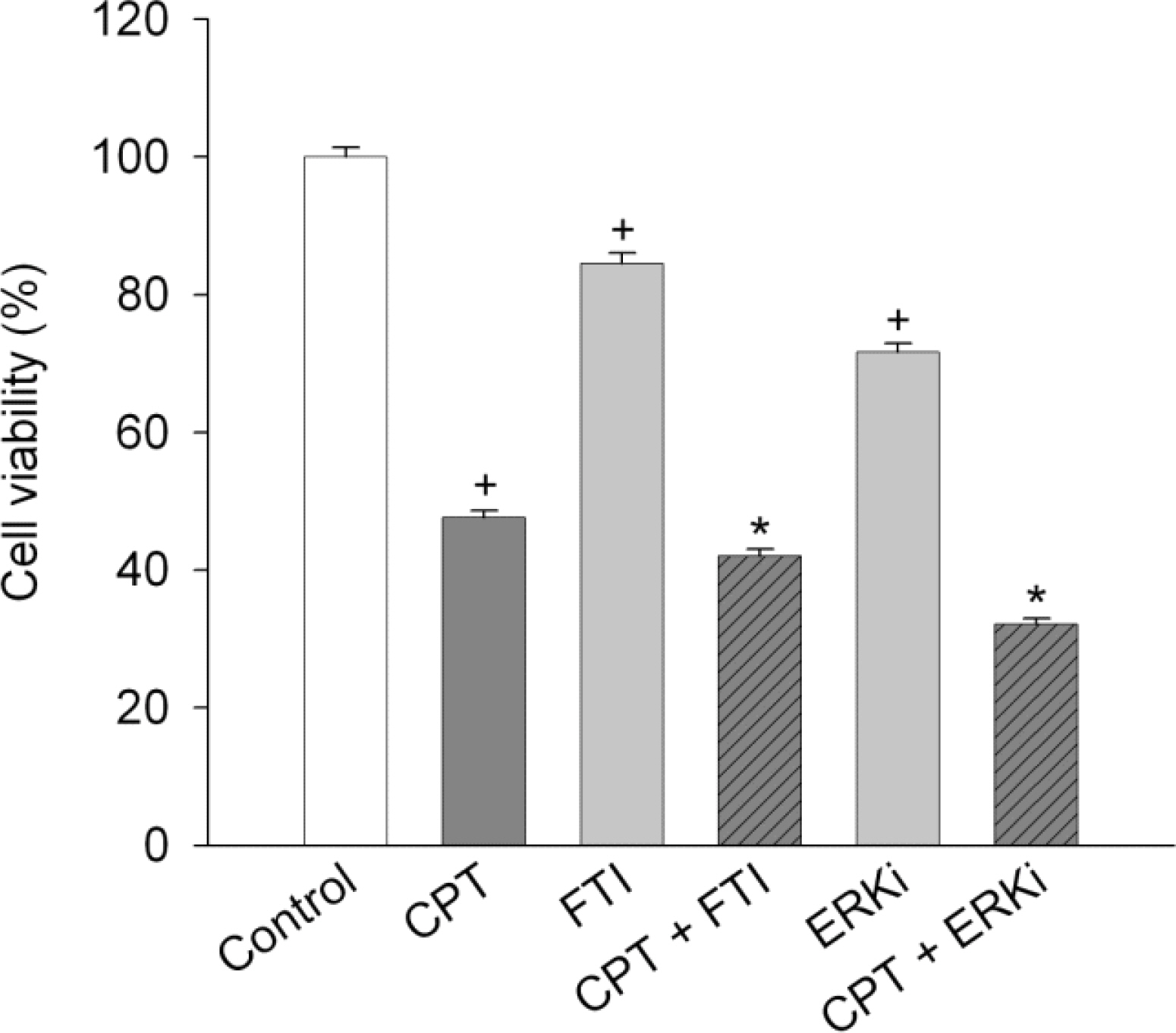
- Functional defects in mitochondria are involved in the induction of cell death in cancer cells. We assessed the toxic effect of camptothecin against the human cervical and uterine tumor cell line SiHa with respect to the mitochondria-mediated cell death process, and examined the combined effect of camptothecin and anticancer drugs. Camptothecin caused apoptosis in SiHa cells by inducing mitochondrial membrane permeability changes that lead to the loss of mitochondrial membrane potential, decreased Bcl-2 levels, cytochrome c release, caspase-3 activation, formation of reactive oxygen species and depletion of GSH. Combination of camptothecin with other anticancer drugs (carboplatin, paclitaxel, doxorubicin and mitomycin c) or signaling inhibitors (farnesyltransferase inhibitor and ERK inhibitor) did not enhance the camptothecin-induced cell death and caspase-3 activation. These results suggest that camptothecin may cause cell death in SiHa cells by inducing changes in mitochondrial membrane permeability, which leads to cytochrome c release and activation of caspase-3. This effect is also associated with increased formation of reactive oxygen species and depletion of GSH. Combination with other anticancer drugs (or signaling inhibitors) does not appear to increase the anti-tumor effect of camptothecin against SiHa cells, but rather may reduce it. Combination of camptothecin with other anticancer drugs does not seem to provide a benefit in the treatment of cervical and uterine cancer compared with camptothecin monotherapy.
Keyword
MeSH Terms
-
Apoptosis
Camptothecin
Carcinoma, Squamous Cell
Caspase 3
Cell Death
Cell Line
Cell Line, Tumor
Cytochromes c
Doxorubicin
Humans
Membrane Potential, Mitochondrial
Mitochondria
Mitochondrial Membranes
Mitomycin
Paclitaxel
Permeability
Reactive Oxygen Species
Uterine Neoplasms
Camptothecin
Caspase 3
Cytochromes c
Doxorubicin
Mitomycin
Paclitaxel
Reactive Oxygen Species
Figure
Reference
-
Ackermann S., Beckmann MW., Thiel F., Bogenrieder T. Topotecan in cervical cancer. Int J Gynecol Cancer. 17:1215–1223. 2007.
ArticleArbuck SG., Takimoto CH. An overview of topoisomerase I-targeting agents. Semin Hematol. 35(Suppl 4):3–12. 1998.Armstrong JS. Mitochondria: a target for cancer therapy. Br J Pharmacol. 147:239–248. 2006.
ArticleBerthier A., Lemaire-Ewing S., Prunet C., Monier S., Athias A., Bessede G., Pais de Barros JP., Laubriet A., Gambert P., Nèel D. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 11:97–905. 2004.
ArticleBlum R., Jacob-Hirsch J., Rechavi G., Kloog Y. Suppression of survivin expression in glioblastoma cells by the Ras inhibitor farnesylthiosalicylic acid promotes caspase-dependent apoptosis. Mol Cancer Ther. 5:2337–2347. 2006.
ArticleBrown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1411:351–369. 1999.
ArticleChandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 29:323–333. 2000.
ArticleChang F., Steelman LS., Shelton JG., Lee JT., Navolanic PM., Blalock WL., Franklin R., McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway. Int J Oncol. 22:469–480. 2003.Constantini PC., Chernyak BC., Petronilli V., Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 271:6746–6751. 1996.Crow MT., Mani K., Nam YJ., Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 95:957–970. 2004.
ArticleDias N., Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 70:1–12. 2005.
ArticleFaivre S., Djelloul S., Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 33:407–420. 2006.
ArticleFerreira CG., Span SW., Peters GJ., Kruyt FA., Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 60:7133–7141. 2000.Fleury C., Mignotte B., Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 84:131–141. 2002.
ArticleFu W., Luo H., Parthasarathy S., Mattson MP. Catecholamines potentiate amyloid β-peptide neurotoxicity: involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol Dis. 5:229–243. 1998.
ArticleHall AG. The role of glutathione in the regulation of apoptosis. Eur J Clin Invest. 29:238–245. 1999.
ArticleHong JS., Ko HH., Han ES., Lee CS. Inhibition of bleomycin-induced cell death in rat alveolar macrophages and human lung epithelial cells by ambroxol. Biochem Pharmacol. 66:1297–1306. 2003.
ArticleHuang X., Kurose A., Tanaka T., Traganos F., Dai W., Darzynkiewicz Z. Activation of ATM and histone H2AX phosphorylation induced by mitoxantrone but not by topotecan is prevented by the antioxidant N-acetyl-L-cysteine. Cancer Biol Ther. 5:959–964. 2006.
ArticleKim R., Emi M., Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 57:545–553. 2006.
ArticleKobayashi T., Sawa H., Morikawa J., Jhang W., Shiku H. Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Jpn J Cancer Res. 91:1264–1268. 2000.Lee CS., Kim YJ., Lee MS., Han ES., Lee SJ. 18β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 83:481–489. 2008.
ArticleLee CS., Park SY., Ko HH., Han ES. Effect of change in cellular GSH levels on mitochondrial damage and cell viability loss due to mitomycin c in small cell lung cancer cells. Biochem Pharmacol. 68:1857–1867. 2004.
ArticleMignotte B., Vayssière JL. Mitochondria and apoptosis. Eur J Biochem. 252:1–15. 1998.
ArticleMosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.
ArticleNitiss JL., Wang JC. Mechanisms of cell killing by drugs that trap covalent complexes between DNA topoisomerases and DNA. Mol Pharmacol. 50:1095–1102. 1996.Pirnia F., Schneider E., Betticher DC., Borner M. Mitomycin c induces apoptosis and caspase-8 and −9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ. 9:905–914. 2002.
ArticlePorter SE., Champoux JJ. The basis for camptothecin enhancement of DNA breakage by eukaryotic topoisomerase I. Nucleic Acids Res. 17:8521–8532. 1989.
ArticlePritsos CA., Briggs LA., Gustafson DL. A new cellular target for mitomycin c: a case for mitochondrial DNA. Oncol Res. 9:333–337. 1997.Sane AT., Cantin AM., Paquette B., Wagner JR. Ascorbate modulation of H2O2 and camptothecin-induced cell death in Jurkat cells. Cancer Chemother Pharmacol. 54:315–321. 2004.
ArticleShao RG., Cao CX., Zhang H., Kohn KW., Wold MS., Pommier Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA: DNA-PK complexes. EMBO J. 18:1397–1406. 1999.Timur M., Akbas SH., Ozben T. The effect of Topotecan on oxidative stress in MCF-7 human breast cancer cell line. Acta Biochim Pol. 52:897–902. 2005.
ArticleTsao YP., Russo A., Nyamuswa G., Silber R., Liu LF. Interaction between replication forks and topoisomerase I-DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer Res. 53:5908–5914. 1993.Van Klaveren RJ., Hoet PHM., Pype JL., Demedts M., Nemery B. Increase in γ-glutamyltransferase by glutathione depletion in rat type II pneumocytes. Free Radic Biol Med. 22:525–534. 1997.
ArticleWang P., Song JH., Song DK., Zhang J., Hao C. Role of death receptor and mitochondrial pathways in conventional chemotherapy drug induction of apoptosis. Cell Signal. 18:1528–1535. 2006.
ArticleWenzel U., Nickel A., Kuntz S., Daniel H. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis. 25:703–712. 2004.
ArticleXia S., Rosen EM., Laterra J. Sensitization of glioma cells to Fas-dependent apoptosis by chemotherapy-induced oxidative stress. Cancer Res. 65:5248–5255. 2005.
ArticleYang X., Zhang C., Ying M., Yang B., He Q. Antiproliferation in human EA.hy926 endothelial cells and inhibition of VEGF expression in PC-3 cells by topotecan. Pharmazie. 62:534–538. 2007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Glutathione on Cisplatin-Induced Cytotoxicity In Human Cervical Cancer Cell Lines
- Effect of Topotecan in Combication with Other Antitumor Drugs in Vitro
- cDNA Microarray Expression Analysis in HPV-Infected Uterine Cervical Cancer Cell Line
- A Case of Ovarian Squamous Cell Carcinoma in a Patient with Microinvasive Squamous Cell Carcinoma of Cervix
- Modulation of Cytotoxicity by Nitric Oxide Donors during Treatment of Glioma with Anticancer Drugs