J Korean Med Sci.
2014 Nov;29(11):1555-1561. 10.3346/jkms.2014.29.11.1555.
A Rat Model of Striatonigral Degeneration Generated by Simultaneous Injection of 6-Hydroxydopamine into the Medial Forebrain Bundle and Quinolinic Acid into the Striatum
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. srjeon@amc.seoul.kr
- 2Department of Computer Science and Engineering, University of Notre Dame, Notre Dame, IN 46556, USA.
- KMID: 2069939
- DOI: http://doi.org/10.3346/jkms.2014.29.11.1555
Abstract
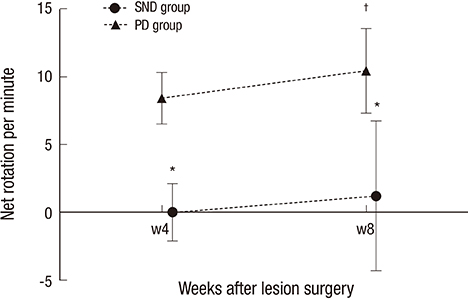
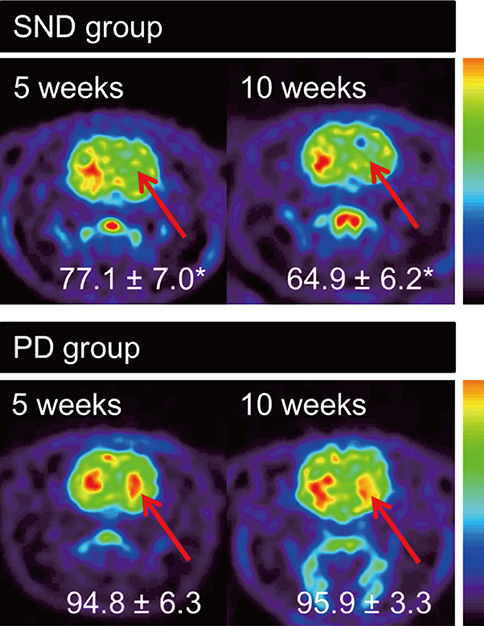
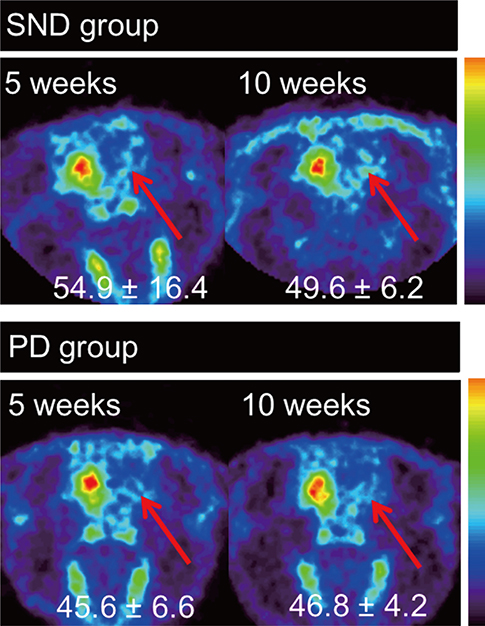
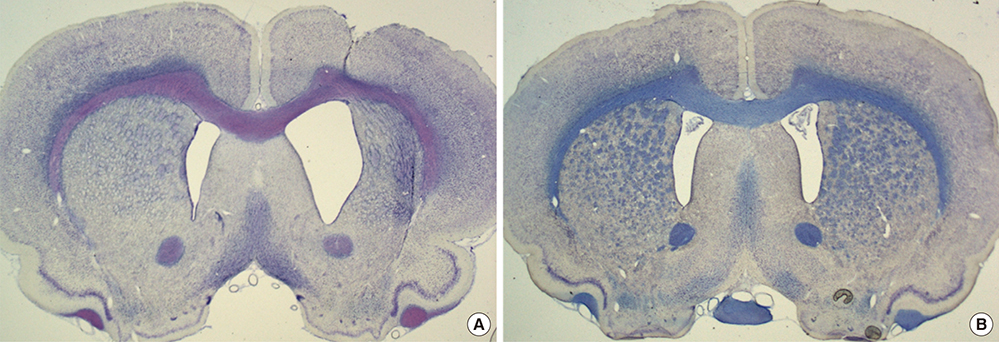
- A double toxin-double lesion strategy is well-known to generate a rat model of striatonigral degeneration (SND) such as multiple system atrophy-parkinsonian type. However, with this model it is difficult to distinguish SND from Parkinson's disease (PD). In this study, we propose a new rat model of SND, which is generated by simultaneous injection of 6-hydroxydopamine into the medial forebrain bundle and quinolinic acid into the striatum. Stepping tests performed 30 min after intraperitoneal L-dopa administration at 6 weeks post-surgery revealed an L-dopa response in the PD group but not the SND group. Apomorphine-induced rotation tests revealed no rotational bias in the SND group, which persisted for 2 months, but contralateral rotations in the PD group. MicroPET scans revealed glucose hypometabolism and dopamine transporter impairment on the lesioned striatum in the SND group. Tyrosine hydroxylase immunostaining in the SND group revealed that 74.7% of nigral cells on the lesioned side were lost after lesion surgery. These results suggest that the proposed simultaneous double toxin-double lesion method successfully created a rat model of SND that had behavioral outcomes, multitracer microPET evaluation, and histological aspects consistent with SND pathology. This model will be useful for future study of SND.
Keyword
MeSH Terms
-
Animals
Apomorphine/pharmacology
Behavior, Animal/drug effects
Corpus Striatum/drug effects/pathology
Disease Models, Animal
Dopamine Plasma Membrane Transport Proteins/metabolism
Glucose/metabolism
Injections, Intraperitoneal
Levodopa/pharmacology
Male
Medial Forebrain Bundle/drug effects/pathology
Oxidopamine/*toxicity
Parkinson Disease/metabolism/pathology
Positron-Emission Tomography
Quinolinic Acid/*toxicity
Rats
Rats, Wistar
Striatonigral Degeneration/*chemically induced/metabolism/pathology
Touch/drug effects
Apomorphine
Dopamine Plasma Membrane Transport Proteins
Glucose
Levodopa
Oxidopamine
Quinolinic Acid
Figure
Reference
-
1. Fernagut PO, Tison F. Animal models of multiple system atrophy. Neuroscience. 2012; 211:77–82.2. Kaindlstorfer C, Garcia J, Winkler C, Wenning GK, Nikkhah G, Döbrössy MD. Behavioral and histological analysis of a partial double-lesion model of parkinson-variant multiple system atrophy. J Neurosci Res. 2012; 90:1284–1295.3. Stefanova N, Bücke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009; 8:1172–1178.4. Adams RD, Vanbogaert L, Vandereecken H. Striato-nigral degeneration. J Neuropathol Exp Neurol. 1964; 23:584–608.5. Churchyard A, Donnan GA, Hughes A, Howells DW, Woodhouse D, Wong JY, Kalnins RM, Mendelsohn FA, Paxinos G. Dopa resistance in multiple-system atrophy: loss of postsynaptic D2 receptors. Ann Neurol. 1993; 34:219–226.6. Fernagut PO, Ghorayeb I, Diguet E, Tison F. In vivo models of multiple system atrophy. Mov Disord. 2005; 20:S57–S63.7. Stefanova N, Tison F, Reindl M, Poewe W, Wenning GK. Animal models of multiple system atrophy. Trends Neurosci. 2005; 28:501–506.8. Wenning GK, Granata R, Laboyrie PM, Quinn NP, Jenner P, Marsden CD. Reversal of behavioural abnormalities by fetal allografts in a novel rat model of striatonigral degeneration. Mov Disord. 1996; 11:522–532.9. Buisson A, Pateau V, Plotkine M, Boulu RG. Nigrostriatal pathway modulates striatum vulnerability to quinolinic acid. Neurosci Lett. 1991; 131:257–259.10. Ghorayeb I, Puschban Z, Fernagut PO, Scherfler C, Rouland R, Wenning GK, Tison F. Simultaneous intrastriatal 6-hydroxydopamine and quinolinic acid injection: a model of early-stage striatonigral degeneration. Exp Neurol. 2001; 167:133–147.11. Venero JL, Romero-Ramos M, Revuelta M, Machado A, Cano J. Intrastriatal quinolinic acid injections protect against 6-hydroxydopamine-induced lesions of the dopaminergic nigrostriatal system. Brain Res. 1995; 672:153–158.12. Yoon HH, Lee CS, Hong SH, Min J, Kim YH, Hwang O, Jeon SR. Evaluation of a multiple system atrophy model in rats using multitracer microPET. Acta Neurochir (Wien). 2012; 154:935–940.13. Paillé V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkinson's disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007; 22:533–539.14. Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, Poewe W, Wenning GK. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000; 95:377–388.15. Kim JS, Lee JS, Im KC, Kim SJ, Kim SY, Lee DS, Moon DH. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007; 48:1527–1535.16. Hwang O, Baker H, Gross S, Joh TH. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998; 28:140–153.17. Schober A. Classic toxin-induced animal models of Parkinsons disease: 6-OHDA and MPTP. Cell Tissue Res. 2004; 318:215–224.18. Alexi T, Venero JL, Hefti F. Protective effects of neurotrophin-4/5 and transforming growth factor-alpha on striatal neuronal phenotypic degeneration after excitotoxic lesioning with quinolinic acid. Neuroscience. 1997; 78:73–86.19. Moresco RM, Lavazza T, Belloli S, Lecchi M, Pezzola A, Todde S, Matarrese M, Carpinelli A, Turolla E, Zimarino V, et al. Quinolinic acid induced neurodegeneration in the striatum: a combined in vivo and in vitro analysis of receptor changes and microglia activation. Eur J Nucl Med Mol Imaging. 2008; 35:704–715.20. Buisson A, Callebert J, Mathieu E, Plotkine M, Boulu RG. Striatal protection induced by lesioning the substantia nigra of rats subjected to focal ischemia. J Neurochem. 1992; 59:1153–1157.21. Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009; 4:e6344.22. Scherfler C, Puschban Z, Ghorayeb I, Goebel GP, Tison F, Jellinger K, Poewe W, Wenning GK. Complex motor disturbances in a sequential double lesion rat model of striatonigral degeneration (multiple system atrophy). Neuroscience. 2000; 99:43–54.23. Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995; 15:3863–3875.24. Köllensperger M, Stefanova N, Pallua A, Puschban Z, Dechant G, Hainzer M, Reindl M, Poewe W, Nikkhah G, Wenning GK. Striatal transplantation in a rodent model of multiple system atrophy: effects on L-Dopa response. J Neurosci Res. 2009; 87:1679–1685.25. Köllensperger M, Stefanova N, Reindl M, Poewe W, Wenning GK. Loss of dopaminergic responsiveness in a double lesion rat model of the Parkinson variant of multiple system atrophy. Mov Disord. 2007; 22:353–358.26. Shear DA, Dong J, Gundy CD, Haik-Creguer KL, Dunbar GL. Comparison of intrastriatal injections of quinolinic acid and 3-nitropropionic acid for use in animal models of Huntington's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1998; 22:1217–1240.27. Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berl). 1999; 146:42–48.28. Ramaswamy S, McBride JL, Kordower JH. Animal models of Huntington's disease. ILAR J. 2007; 48:356–373.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impaired Voluntary Wheel Running Behavior in the Unilateral 6-Hydroxydopamine Rat Model of Parkinson's Disease
- Relationship between Microglial Activation and Dopaminergic Neuronal Loss in 6-OHDA-induced Parkinsonian Animal Model
- An Autoradiographic Study on the Rat Neostriatal Dopamine Receptor Changes after 6-hydroxydopamine Injection into the Medial Prefrontal Cortex
- Differentiation of Rat Neural Stem Cells Following Transplantation in the Brain of Huntington's Disease Rat Model
- Effects of Fetal Nondopaminergic Cortical Tissue Transplantation in the Rat Parkinsonian Model