J Cardiovasc Ultrasound.
2014 Dec;22(4):189-195. 10.4250/jcu.2014.22.4.189.
Myocardial Mechanics in a Rat Model with Banding and Debanding of the Ascending Aorta
- Affiliations
-
- 1Division of Cardiology, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Division of Cardiology, St. Paul's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. choej4oct@gmail.com
- 3Department of Thoracic and Cardiovascular Surgery, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. phenix@catholic.ac.kr
- 4The University of Debrecen Medical and Health Science Center, Debrecen, Hungary.
- 5Clinical Research Institute, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6Department of Pathology, Hwasun Hospital, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1980422
- DOI: http://doi.org/10.4250/jcu.2014.22.4.189
Abstract
- BACKGROUND
Aortic banding and debanding models have provided useful information on the development and regression of left ventricular hypertrophy (LVH). In this animal study, we aimed to evaluate left ventricular (LV) deformation related to the development and regression of LVH.
METHODS
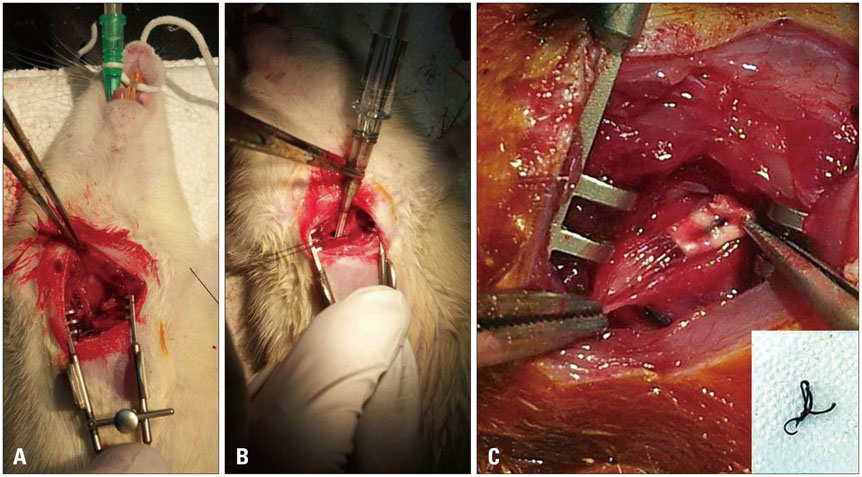
Minimally invasive ascending aorta banding was performed in rats (10 Sprague Dawley rats, 7 weeks). Ten rats underwent a sham operation. Thirty-five days later, the band was removed. Echocardiographic and histopathologic analysis was assessed at pre-banding, 35 days of banding and 14 days of debanding.
RESULTS
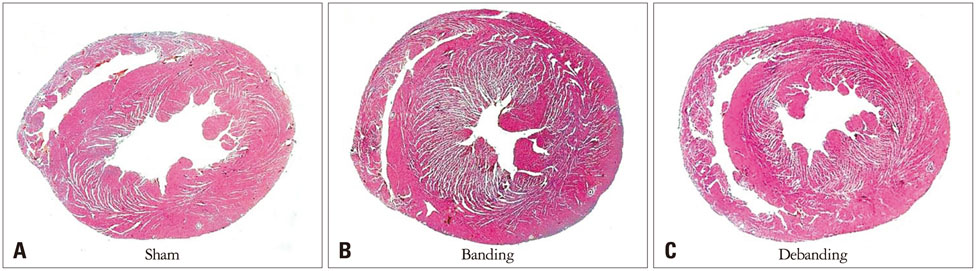
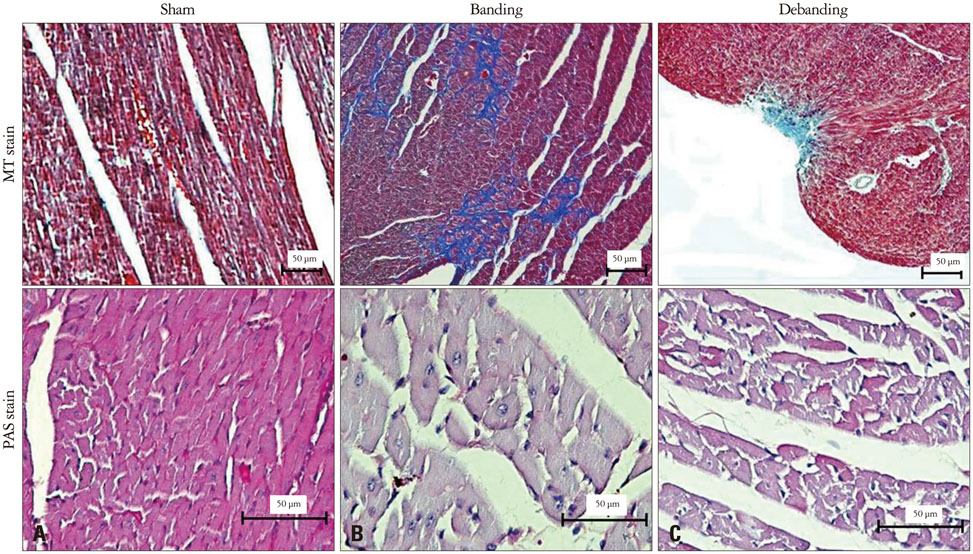
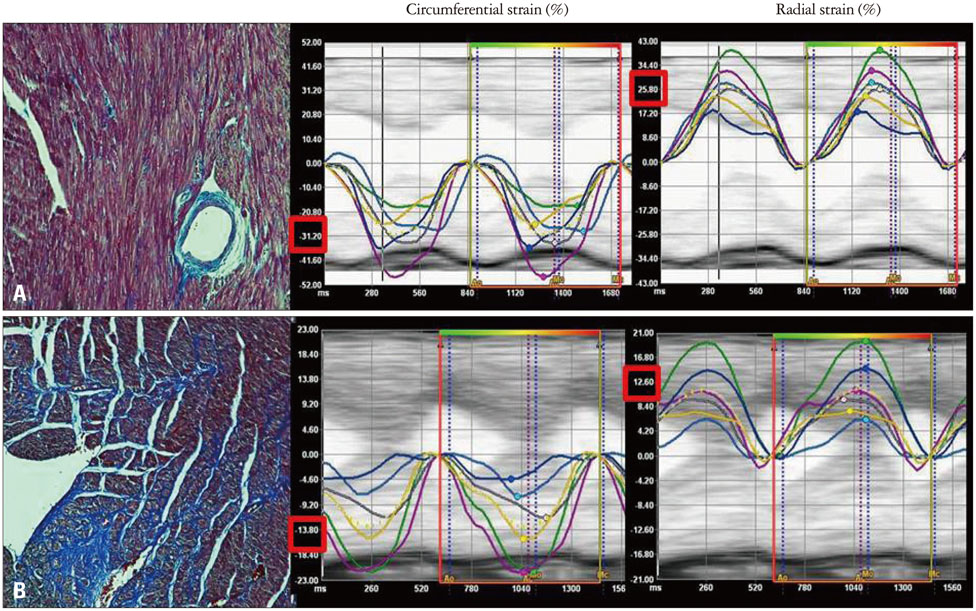
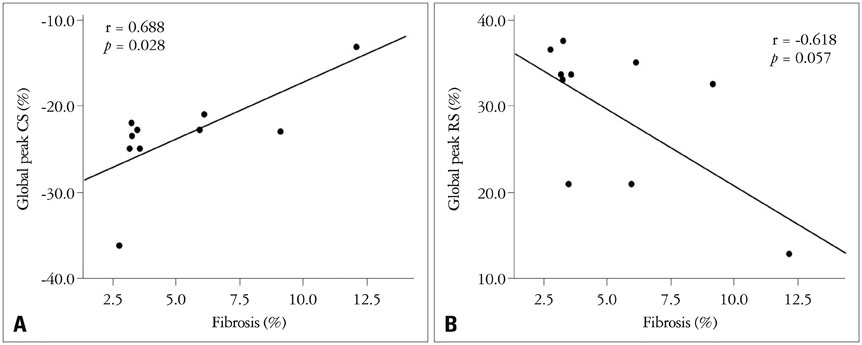
Banding of the ascending aorta created an expected increase in the aortic velocity and gradient, which normalized with the debanding procedure. Pressure overload resulted in a robust hypertrophic response as assessed by gross and microscopic histology, transthoracic echocardiography [heart weight/tibia length (g/m); 21.0 +/- 0.8 vs. 33.2 +/- 2.0 vs. 26.6 +/- 2.8, p < 0.001]. The circumferential (CS) and radial strains were not different between the groups. However, there were significant differences in the degree of fibrosis according to the banding status (fibrosis; 0.10 +/- 0.20% vs. 5.26 +/- 3.12% vs. 4.03 +/- 3.93%, p = 0.003), and global CS showed a significant correlation with the degree of myocardial fibrosis in this animal model (r = 0.688, p = 0.028).
CONCLUSION
In this animal study, simulating a severe LV pressure overload state, a significant increase in the LV mass index did not result in a significant reduction in the LV mechanical parameters. The degree of LV fibrosis, which developed with pressure overload, was significantly related to the magnitude of left ventricular mechanics.
MeSH Terms
Figure
Reference
-
1. Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ. American Heart Association Council on Basic Cardiovascular Sciences. Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012; 111:131–150.2. Gao XM, Kiriazis H, Moore XL, Feng XH, Sheppard K, Dart A, Du XJ. Regression of pressure overload-induced left ventricular hypertrophy in mice. Am J Physiol Heart Circ Physiol. 2005; 288:H2702–H2707.
Article3. Bjørnstad JL, Skrbic B, Sjaastad I, Bjørnstad S, Christensen G, Tønnessen T. A mouse model of reverse cardiac remodelling following bandingdebanding of the ascending aorta. Acta Physiol (Oxf). 2012; 205:92–102.
Article4. Stansfield WE, Rojas M, Corn D, Willis M, Patterson C, Smyth SS, Selzman CH. Characterization of a model to independently study regression of ventricular hypertrophy. J Surg Res. 2007; 142:387–393.
Article5. Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Aronson D, Reisner SA, Rakowski H, Bolotin G, Agmon Y. Relation of myocardial mechanics in severe aortic stenosis to left ventricular ejection fraction and response to aortic valve replacement. Am J Cardiol. 2011; 107:1052–1057.
Article6. Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Reisner SA, Rakowski H, Bolotin G, Agmon Y. Differential effects of afterload on left ventricular long- and short-axis function: insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am Heart J. 2009; 158:540–545.
Article7. Schattke S, Baldenhofer G, Prauka I, Zhang K, Laule M, Stangl V, Sanad W, Spethmann S, Borges AC, Baumann G, Stangl K, Knebel F. Acute regional improvement of myocardial function after interventional transfemoral aortic valve replacement in aortic stenosis: a speckle tracking echocardiography study. Cardiovasc Ultrasound. 2012; 10:15.
Article8. Bauer F, Eltchaninoff H, Tron C, Lesault PF, Agatiello C, Nercolini D, Derumeaux G, Cribier A. Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation. 2004; 110:1473–1476.
Article9. Peng Y, Popovic ZB, Sopko N, Drinko J, Zhang Z, Thomas JD, Penn MS. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2009; 297:H811–H820.
Article10. Popović ZB, Benejam C, Bian J, Mal N, Drinko J, Lee K, Forudi F, Reeg R, Greenberg NL, Thomas JD, Penn MS. Speckle-tracking echocardiography correctly identifies segmental left ventricular dysfunction induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007; 292:H2809–H2816.
Article11. Azam S, Desjardins CL, Schluchter M, Liner A, Stelzer JE, Yu X, Hoit BD. Comparison of velocity vector imaging echocardiography with magnetic resonance imaging in mouse models of cardiomyopathy. Circ Cardiovasc Imaging. 2012; 5:776–781.
Article12. Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in Mice. Curr Protoc Mouse Biol. 2011; 1:71–83.
Article13. Täng MS, Redfors B, Shao Y, Omerovic E. Velocity vector imaging fails to quantify regional myocardial dysfunction in a mouse model of isoprenaline-induced cardiotoxicity. Echocardiography. 2012; 29:818–826.
Article14. Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005; 112:1136–1144.
Article15. Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989; 79:744–755.
Article16. Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011; 57:891–903.
Article17. Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation. 2002; 105:1602–1608.
Article18. Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014; 7:11–19.
Article19. Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010; 106:47–57.
Article20. Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011; 108:908–916.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The relationship between miRNA-26b and connective tissue growth factor in rat models of aortic banding and debanding
- An Experimental Study of Percutaneously Adjustable Pulmonary Artery Banding Device
- Sudden Cardiac Death from Acute Myocardial Infarction Caused by Unruptured Ascending Aortic Aneurysm Involving the Sinus of Valsalva: An Autopsy Case
- A Case of a Aneurysm of the Ascending Aorta Following Open Heart Surgery
- Delayed Ascending Aorta Replacement in Blunt Chest Trauma with Aortic Injury