J Bone Metab.
2012 Nov;19(2):139-145. 10.11005/jbm.2012.19.2.139.
Bisphosphonate-induced Severe Hypocalcemia: A Case Report
- Affiliations
-
- 1Department of Internal Medicine, Daegu Fatima Hospital, Daegu, Korea. dhlee@fatima.or.kr
- KMID: 1975988
- DOI: http://doi.org/10.11005/jbm.2012.19.2.139
Abstract
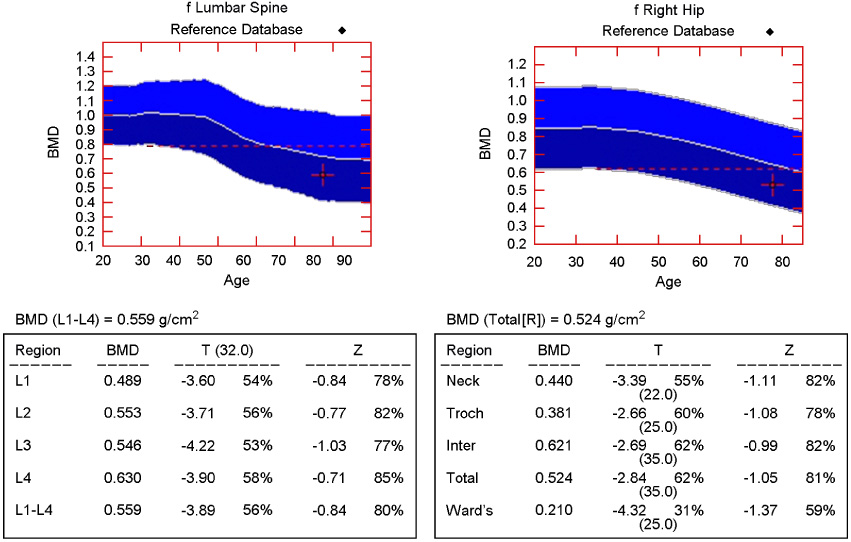
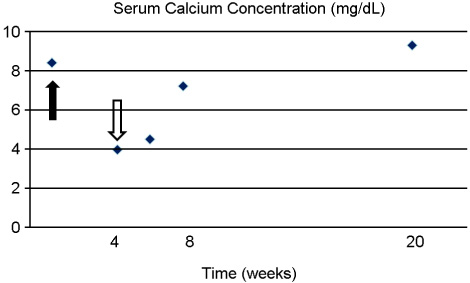
- Bisphosphonate generally seems to be safe, but hypocalcemia may occasionally develop in the course of bisphosphonate treatment. Hypocalcemia induced by bisphosphonate is usually mild and asymptomatic, but unrecognized or poorly treated hypocalcemia can lead to life-threatening state. A 78-year-old woman who had a history of hip arthroplasty and intravenous zoledronate treatment for femur neck fracture was presented to emergency department with altered mental status. It turned out that her symptom was due to severe hypocalcemia which was caused by intravenous zoledronate treatment. She also had renal dysfunction. She was treated by intravenous calcium gluconate and calcitriol administration. This case supports the need for evaluation of renal dysfunction, vitamin D deficiency and parathyroid gland dysfunction before bisphosphonate treatment and accurate monitoring of plasma calcium and creatinine levels. In addition, vitamin D and calcium supply during treatment with bisphosphonate is mandatory.
Keyword
MeSH Terms
-
Aged
Arthroplasty
Calcitriol
Calcium
Calcium Gluconate
Creatinine
Diphosphonates
Emergencies
Female
Femoral Neck Fractures
Gluconates
Hip
Humans
Hypocalcemia
Imidazoles
Osteoporosis
Parathyroid Glands
Plasma
Vitamin D
Vitamin D Deficiency
Calcitriol
Calcium
Calcium Gluconate
Creatinine
Diphosphonates
Gluconates
Imidazoles
Vitamin D
Figure
Reference
-
1. Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens). 2009. 8:96–110.
Article2. Hamdy RC. Zoledronic acid: clinical utility and patient considerations in osteoporosis and low bone mass. Drug Des Devel Ther. 2010. 4:321–335.
Article3. Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003. 21:3150–3157.
Article4. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002. 94:1458–1468.
Article5. Peter R, Mishra V, Fraser WD. Severe hypocalcaemia after being given intravenous bisphosphonate. BMJ. 2004. 328:335–336.
Article6. Jodrell DI, Iveson TJ, Smith IE. Symptomatic hypocalcaemia after treatment with high-dose aminohydroxypropylidene diphosphonate. Lancet. 1987. 1:622.
Article7. Chesnut CH 3rd, McClung MR, Ensrud KE, et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med. 1995. 99:144–152.
Article8. Mortensen L, Charles P, Bekker PJ, et al. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998. 83:396–402.
Article9. Conte PF, Latreille J, Mauriac L, et al. The Aredia Multinational Cooperative Group. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. J Clin Oncol. 1996. 14:2552–2559.
Article10. Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005. 23:3314–3321.
Article11. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007. 357:1799–1809.
Article12. Maalouf NM, Heller HJ, Odvina CV, et al. Bisphosphonate-induced hypocalcemia: report of 3 cases and review of literature. Endocr Pract. 2006. 12:48–53.
Article13. Hanamura M, Iwamoto T, Soga N, et al. Risk factors contributing to the development of hypocalcemia after zoledronic acid administration in patients with bone metastases of solid tumor. Biol Pharm Bull. 2010. 33:721–724.
Article14. Malluche H, Faugere MC. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int. 1990. 38:193–211.
Article15. Courtney AE, Maxwell AP. Chronic kidney disease and bisphosphonate treatment: are prescribing guidelines unnecessarily restrictive? Postgrad Med J. 2009. 85:327–330.
Article16. Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007. 22:503–508.
Article17. Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005. 20:2105–2115.
Article18. Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009. 4:221–233.
Article19. Miller PD. The kidney and bisphosphonates. Bone. 2011. 49:77–81.
Article20. Couttenye MM, D'Haese PC, Deng JT, et al. High prevalence of adynamic bone disease diagnosed by biochemical markers in a wide sample of the European CAPD population. Nephrol Dial Transplant. 1997. 12:2144–2150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversible Heart Failure and Rhabdomyolysis Caused by Primary Hypoparathyroidism during Lactation
- A Case of Hypocalcemia-Induced Dilated Cardiomyopathy
- Bisphosphonate: An Invaluable Medication or Abandoned Acid?
- Takotsubo Cardiomyopathy Associated with Severe Hypocalcemia Secondary to Idiopathic Hypoparathyroidism
- Oral Bisphosphonate Related Osteonecrosis of the Jaws: A case report