Diabetes Metab J.
2013 Oct;37(5):358-364. 10.4093/dmj.2013.37.5.358.
Effect of Treadmill Exercise on Interleukin-15 Expression and Glucose Tolerance in Zucker Diabetic Fatty Rats
- Affiliations
-
- 1Health and Exercise Science Laboratory, The Institute of Sports Science, Seoul National University, Seoul, Korea. songw3@snu.ac.kr
- 2Department of Sport, Kyungil University College of Arts and Sports, Gyeongsan, Korea.
- 3Department of Anatomy and Cell Biology, Research Institute for Veterinary Science, Seoul National University College of Veterinary Medicine, Seoul, Korea.
- 4Institute on Aging, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1965983
- DOI: http://doi.org/10.4093/dmj.2013.37.5.358
Abstract
- BACKGROUND
Interleukin-15 (IL-15), a well-known myokine, is highly expressed in skeletal muscle and is involved in muscle-fat crosstalk. Recently, a role of skeletal muscle-derived IL-15 in the improvement of glucose homeostasis and insulin sensitivity has been proposed. However, little is known regarding the influence of endurance training on IL-15 expression in type 2 diabetic skeletal muscles. We investigated the effect of endurance exercise training on glucose tolerance and IL-15 expression in skeletal muscles using type 2 diabetic animal models.
METHODS
Male Zucker diabetic fatty (ZDF) and ZDF lean control (ZLC) rats were randomly divided into three groups: sedentary ZLC, sedentary ZDF (ZDF-Con), and exercised ZDF (ZDF-Ex). The ZDF-Ex rats were forced to run a motor-driven treadmill for 60 minutes once a day 5 times per week for 12 weeks. Intraperitoneal glucose tolerance test (IPGTT) was performed after 12 weeks. Expression of IL-15 was measured using ELISA in extracted soleus (SOL) and gastrocnemius medial muscles.
RESULTS
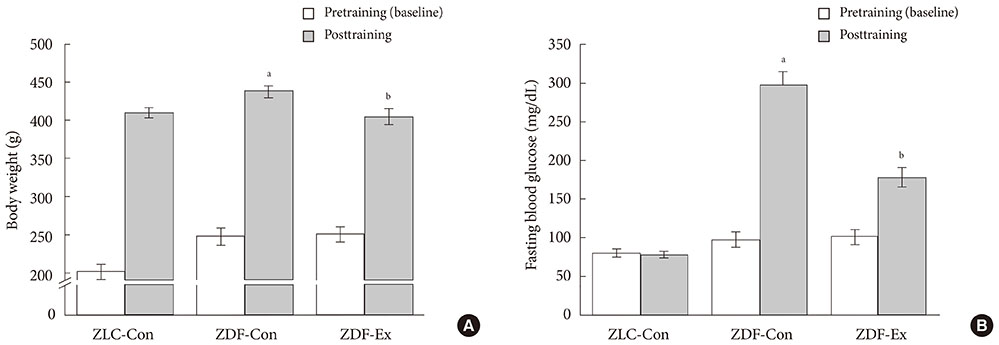
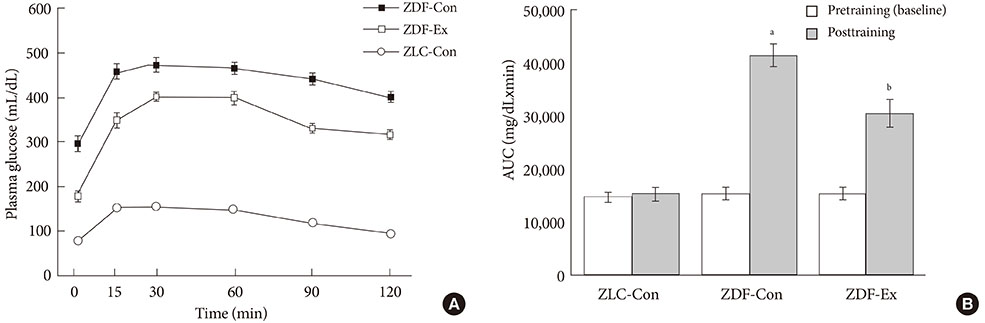
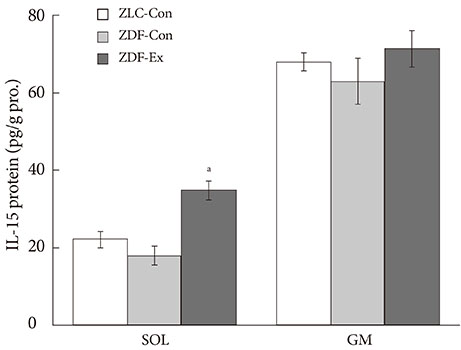
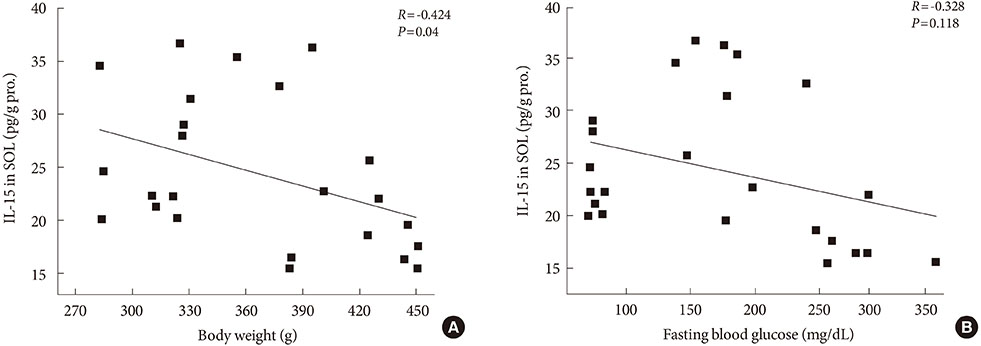
After 12 weeks of treadmill training, reduction of body weight was observed in ZDF-Ex compared to ZDF-Con rats. Glucose tolerance using IPGTT in diabetic rats was significantly improved in ZDF-Ex rats. Furthermore, the expression of IL-15 was significantly increased (P<0.01) only in the SOL of ZDF-Ex rats compared to ZDF-Con. Additionally, IL-15 expression in SOL muscles was negatively correlated with change of body weight (R=-0.424, P=0.04).
CONCLUSION
The present study results suggest that 12 weeks of progressive endurance training significantly improved glucose tolerance with concomitant increase of IL-15 expression in SOL muscles of type 2 diabetic rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007; 103:1093–1098.2. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Alderson MR, Watson JD, Anderson DM, Giri JG. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994; 264:965–968.3. Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995; 136:3669–3672.4. Argiles JM, Lopez-Soriano FJ, Busquets S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov Today. 2009; 14:208–213.5. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002; 280:55–63.6. Busquets S, Figueras M, Almendro V, Lopez-Soriano FJ, Argiles JM. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim Biophys Acta. 2006; 1760:1613–1617.7. Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Muscle wasting in cancer and ageing: cachexia versus sarcopenia. Adv Gerontol. 2006; 18:39–54.8. Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004; 97:2214–2219.9. Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007; 584(Pt 1):305–312.10. Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004; 287:R322–R327.11. Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998; 513(Pt 3):889–894.12. Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol. 2004; 96:1292–1298.13. Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011; 58:211–215.14. DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992; 15:318–368.15. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985; 6:45–86.16. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350.17. Hwang IK, Yi SS, Kim YN, Kim IY, Lee IS, Yoon YS, Seong JK. Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2008; 33:394–400.18. Chang H, Park JY, Suk MH, Lee HJ, Kang HJ, Choi KM, Song W. Comparison of lactate threshold, glucose, and insulin levels between OLETF and LETO rats after all-out exercise. J Sports Sci Med. 2009; 8:381–387.19. Almendro V, Busquets S, Ametller E, Carbo N, Figueras M, Fuster G, Argiles JM, Lopez-Soriano FJ. Effects of interleukin-15 on lipid oxidation: disposal of an oral [(14)C]-triolein load. Biochim Biophys Acta. 2006; 1761:37–42.20. Alvarez B, Carbo N, Lopez-Soriano J, Drivdahl RH, Busquets S, Lopez-Soriano FJ, Argiles JM, Quinn LS. Effects of interleukin-15 (IL-15) on adipose tissue mass in rodent obesity models: evidence for direct IL-15 action on adipose tissue. Biochim Biophys Acta. 2002; 1570:33–37.21. Carbo N, Lopez-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta. 2001; 1526:17–24.22. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab. 2009; 296:E191–E202.23. Barra NG, Chew MV, Holloway AC, Ashkar AA. Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes Metab. 2012; 14:190–193.24. Quinn LS, Anderson BG. Interleukin-15, IL-15 receptor-alpha, and obesity: Concordance of Laboratory Animal and Human Genetic Studies. J Obes. 2011; 2011:456347.25. Lambert CP, Flynn MG, Sullivan DH, Evans WJ. Effects of megestrol acetate on circulating interleukin-15 and interleukin-18 concentrations in healthy elderly men. J Gerontol A Biol Sci Med Sci. 2004; 59:855–858.26. Yang H, Chang J, Chen W, Zhao L, Qu B, Tang C, Qi Y, Zhang J. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine. 2013; 43:579–585.27. Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care. 1994; 17:382–386.28. Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol. 1995; 268(3 Pt 1):E453–E457.29. Nyholm B, Qu Z, Kaal A, Pedersen SB, Gravholt CH, Andersen JL, Saltin B, Schmitz O. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997; 46:1822–1828.30. Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006; 29:895–900.31. Yasuda K, Adachi T, Kikuchi N, Tsujimoto G, Aoki N, Tsuda K, Ishihara A. Effects of running exercise on fibre-type distribution of soleus and plantaris muscles in diabetic Otsuka Long-Evans Tokushima fatty rats. Diabetes Obes Metab. 2006; 8:311–321.32. Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K, Ishihara A. Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol. 2002; 29:1001–1008.33. Adachi T, Kikuchi N, Yasuda K, Anahara R, Gu N, Matsunaga T, Yamamura T, Mori C, Tsujimoto G, Tsuda K, Ishihara A. Fibre type distribution and gene expression levels of both succinate dehydrogenase and peroxisome proliferator-activated receptor-gamma coactivator-1alpha of fibres in the soleus muscle of Zucker diabetic fatty rats. Exp Physiol. 2007; 92:449–455.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of regular physical exercise on glucose uptake in soleus and intravenous glucose tolerance in streptozotocin diabetic rats
- The Preventive Effects of 8 Weeks of Resistance Training on Glucose Tolerance and Muscle Fiber Type Composition in Zucker Rats
- Treadmill exercise prevents diabetes-induced increases in lipid peroxidation and decreases in Cu,Zn-superoxide dismutase levels in the hippocampus of Zucker diabetic fatty rats
- Differential effects of treadmill exercise on cyclooxygenase-2 in the rat hippocampus at early and chronic stages of diabetes
- Effect of Hyperglycemia and Hyperlipidemia on Cardiac Muscle Glycogen Usage during Exercise in Rats