Diabetes Metab J.
2015 Oct;39(5):424-433. 10.4093/dmj.2015.39.5.424.
The Preventive Effects of 8 Weeks of Resistance Training on Glucose Tolerance and Muscle Fiber Type Composition in Zucker Rats
- Affiliations
-
- 1Health and Exercise Science Laboratory, Institute of Sports Science, Seoul National University College of Education, Seoul, Korea. songw3@snu.ac.kr
- 2Laboratory of Developmental Biology and Genomics, BK21 Program for Veterinary Science, Institute for Veterinary Science, Seoul National University College of Veterinary Medicine, Seoul, Korea.
- 3Korea Mouse Phenotyping Center (KMPC), Seoul, Korea.
- 4Institute on Aging, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2174016
- DOI: http://doi.org/10.4093/dmj.2015.39.5.424
Abstract
- BACKGROUND
We investigated the therapeutic effects of resistance training on Zucker rats before and after the onset of diabetes to understand the importance of the timing of exercise intervention. We assessed whether 8 weeks of resistance training ameliorated impaired glucose tolerance and altered muscle fiber type composition in Zucker rats.
METHODS
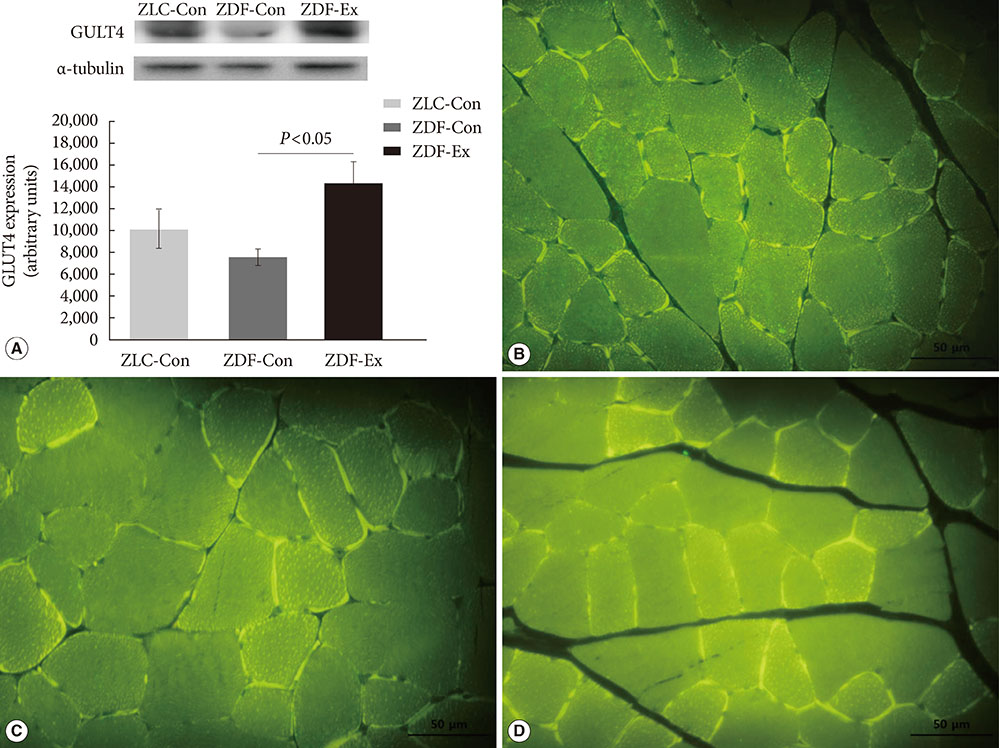
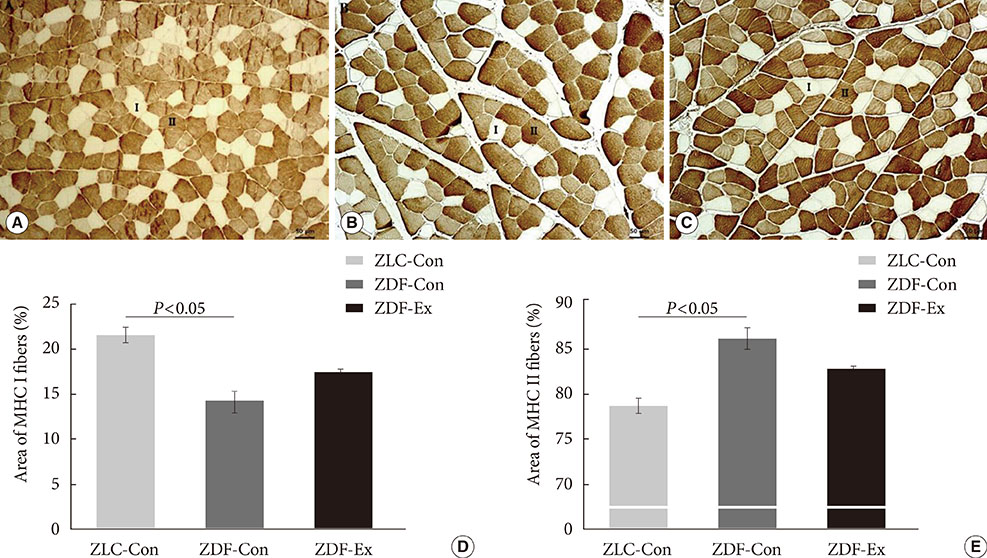
Five-week-old male Zucker rats were divided into Zucker lean control (ZLC-Con), non-exercised Zucker diabetic fatty (ZDF-Con), and exercised Zucker diabetic fatty (ZDF-Ex) groups. The ZDF-Ex rats climbed a ladder three times a week for 8 weeks. Intraperitoneal glucose tolerance tests (IPGTT) were performed on the 1st and 8th weeks of training, and grip strength was measured during the last week. We also measured glucose transporter 4 (GLUT4) expression by Western blot and immunofluorescence. Moreover, immunohistochemistry was performed to assess muscle fiber type composition.
RESULTS
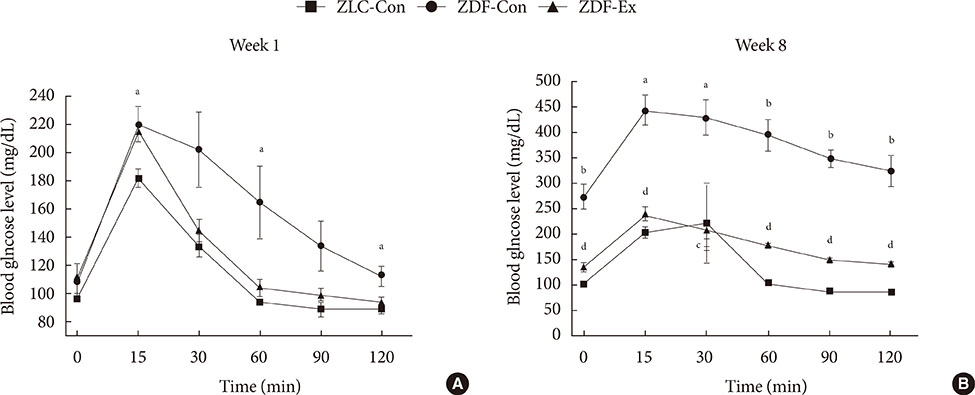
Fasting glucose levels and area under the curve responses to IPGTTs gradually increased as diabetes progressed in the ZDF-Con rats but decreased in the ZDF-Ex rats. Grip strength decreased in the ZDF-Con rats. However, resistance training did not improve grip strength in the ZDF-Ex rats. GLUT4 expression in the ZLC-Con and the ZDF-Con rats did not differ, but it increased in the ZDF-Ex rats. The proportions of myosin heavy chain I and II were lower and higher, respectively, in the ZDF-Con rats compared to the ZLC-Con rats. Muscle fiber type composition did not change in the ZDF-Ex rats.
CONCLUSION
Our results suggest that regular resistance training initiated at the onset of diabetes can improve glucose tolerance and GLUT4 expression without changing muscle morphology in Zucker rats.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Diabetes Mellitus, Type 2
Fasting
Fluorescent Antibody Technique
Glucose Tolerance Test
Glucose Transport Proteins, Facilitative
Glucose*
Hand Strength
Humans
Immunohistochemistry
Male
Myosin Heavy Chains
Rats
Rats, Zucker*
Resistance Training*
Glucose
Glucose Transport Proteins, Facilitative
Myosin Heavy Chains
Figure
Reference
-
1. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006; 368:1681–1688.2. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001; 414:782–787.3. Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002; 90(5A):11G–18G.4. Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis. 2000; 148:231–241.5. Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000; 49:684–688.6. Chalmers GR, Edgerton VR. Single motoneuron succinate dehydrogenase activity. J Histochem Cytochem. 1989; 37:1107–1114.7. Hirofuji C, Nakatani T, Ishihara A, Tanaka M, Itoh K, Itoh M, Katsuta S, Ibata Y. Cell size and succinate dehydrogenase activity of different types of fibers in different regions of the tibialis anterior muscle in mice and rats. Acta Histochem Cytochem. 2000; 33:295–303.8. Nakatani T, Nakashima T, Kita T, Hirofuji C, Itoh K, Itoh M, Ishihara A. Cell size and oxidative enzyme activity of different types of fibers in different regions of the rat plantaris and tibialis anterior muscles. Jpn J Physiol. 2000; 50:413–418.9. Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002; 29:218–222.10. Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity: from gene to form and function. Rev Physiol Biochem Pharmacol. 2003; 146:159–216.11. Jarvis JC, Mokrusch T, Kwende MM, Sutherland H, Salmons S. Fast-to-slow transformation in stimulated rat muscle. Muscle Nerve. 1996; 19:1469–1475.12. Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol (1985). 2001; 90:1137–1157.13. Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001; 115:359–372.14. Roy RR, Talmadge RJ, Fox K, Lee M, Ishihara A, Edgerton VR. Modulation of MHC isoforms in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol (1985). 1997; 83:280–290.15. Carroll TJ, Abernethy PJ, Logan PA, Barber M, McEniery MT. Resistance training frequency: strength and myosin heavy chain responses to two and three bouts per week. Eur J Appl Physiol Occup Physiol. 1998; 78:270–275.16. Sharman MJ, Newton RU, Triplett-McBride T, McGuigan MR, McBride JM, Hakkinen A, Hakkinen K, Kraemer WJ. Changes in myosin heavy chain composition with heavy resistance training in 60- to 75-year-old men and women. Eur J Appl Physiol. 2001; 84:127–132.17. Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol (1985). 1993; 74:911–915.18. Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000; 23:1095–1104.19. Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, Marcus R. Muscle hypertrophy response to resistance training in older women. J Appl Physiol (1985). 1991; 70:1912–1916.20. Houmard JA, O'Neill DS, Zheng D, Hickey MS, Dohm GL. Impact of hyperinsulinemia on myosin heavy chain gene regulation. J Appl Physiol (1985). 1999; 86:1828–1832.21. Gaster M, Staehr P, Beck-Nielsen H, Schroder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001; 50:1324–1329.22. Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990; 259(4 Pt 1):E593–E598.23. Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999; 277(6 Pt 2):R1690–R1696.24. Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000; 49:1092–1095.25. Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol (1985). 2004; 96:1097–1104.26. Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ. Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 2005; 42:162–170.27. Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, Zoppini G, Cevese A, Bonadonna RC, Schena F, Bonora E, Lanza M, Moghetti P. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. 2012; 35:676–682.28. Roden M. Exercise in type 2 diabetes: to resist or to endure? Diabetologia. 2012; 55:1235–1239.29. Hornberger TA Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004; 29:16–31.30. Duncan ND, Williams DA, Lynch GS. Adaptations in rat skeletal muscle following long-term resistance exercise training. Eur J Appl Physiol Occup Physiol. 1998; 77:372–378.31. Ho KW, Roy RR, Tweedle CD, Heusner WW, Van Huss WD, Carrow RE. Skeletal muscle fiber splitting with weight-lifting exercise in rats. Am J Anat. 1980; 157:433–440.32. Klitgaard H. A model for quantitative strength training of hindlimb muscles of the rat. J Appl Physiol (1985). 1988; 64:1740–1745.33. Hall KE, McDonald MW, Grise KN, Campos OA, Noble EG, Melling CW. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced type 1 diabetic rodents. Metabolism. 2013; 62:1485–1494.34. Ibanez J, Izquierdo M, Arguelles I, Forga L, Larrion JL, Garcia-Unciti M, Idoate F, Gorostiaga EM. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005; 28:662–667.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Treadmill Exercise on Interleukin-15 Expression and Glucose Tolerance in Zucker Diabetic Fatty Rats
- Effect of Age on Glucose Metabolism of Skeletal Muscle in Rats
- The effect of regular physical exercise on glucose uptake in soleus and intravenous glucose tolerance in streptozotocin diabetic rats
- Effect of Rhodiola Sachalinensis Administration and Endurance Exercise on Insulin Sensitivity and Expression of Proteins Related with Glucose Transport in Skeletal Muscle of Obese Zucker Rat
- Effects of Resistance Exercise and Fermented Soybean Consumption on Glucose Tolerance and Expressions of Immune Senescence-Related Myokines in Middle-Aged Obese Rats