J Vet Sci.
2015 Mar;16(1):11-16. 10.4142/jvs.2015.16.1.11.
Treadmill exercise prevents diabetes-induced increases in lipid peroxidation and decreases in Cu,Zn-superoxide dismutase levels in the hippocampus of Zucker diabetic fatty rats
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ysyoon@snu.ac.kr
- 2BK21 PLUS Program for Creative Veterinary Science Research, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 3Seocho High School, Seoul 137-952, Korea.
- 4Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 5Institute of Sports Science, Seoul National University, Seoul 151-742, Korea.
- KMID: 2160815
- DOI: http://doi.org/10.4142/jvs.2015.16.1.11
Abstract
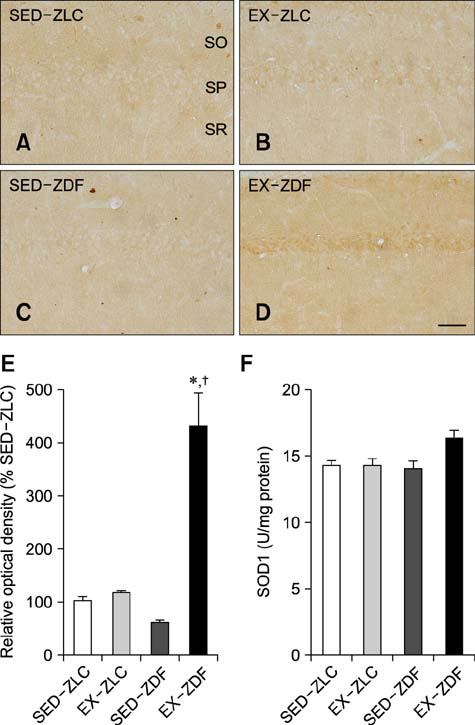
- In the present study, we investigated the effects of treadmill exercise on lipid peroxidation and Cu,Zn-superoxide dismutase (SOD1) levels in the hippocampus of Zucker diabetic fatty (ZDF) rats and lean control rats (ZLC) during the onset of diabetes. At 7 weeks of age, ZLC and ZDF rats were either placed on a stationary treadmill or made to run for 1 h/day for 5 consecutive days at 16~22 m/min for 5 weeks. At 12 weeks of age, the ZDF rats had significantly higher blood glucose levels and body weight than the ZLC rats. In addition, malondialdehyde (MDA) levels in the hippocampus of the ZDF rats were significantly higher than those of the ZLC rats whereas SOD1 levels in the hippocampus of the ZDF rats were moderately decreased. Notably, treadmill exercise prevented the increase of blood glucose levels in ZDF rats. In addition, treadmill exercise significantly ameliorated changes in MDA and SOD1 levels in the hippocampus although SOD activity was not altered. These findings suggest that diabetes increases lipid peroxidation and decreases SOD1 levels, and treadmill exercise can mitigate diabetes-induced oxidative damage in the hippocampus.
Keyword
MeSH Terms
-
Animals
Diabetes Mellitus/enzymology/*metabolism
Female
Gene Expression Regulation, Enzymologic
Genotype
Hippocampus/*enzymology/metabolism
Lipid Peroxidation/*physiology
Male
Malondialdehyde/metabolism
Physical Conditioning, Animal/*physiology
Rats
Rats, Zucker
Superoxide Dismutase/genetics/*metabolism
Malondialdehyde
Superoxide Dismutase
Figure
Reference
-
1. Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer's disease. Behav Brain Res. 2009; 205:265–271.
Article2. Aksu I, Topcu A, Camsari UM, Acikgoz O. Effect of acute and chronic exercise on oxidant-antioxidant equilibrium in rat hippocampus, prefrontal cortex and striatum. Neurosci Lett. 2009; 452:281–285.
Article3. Alipour M, Salehi I, Ghadiri Soufi F. Effect of exercise on diabetes-induced oxidative stress in the rat hippocampus. Iran Red Crescent Med J. 2012; 14:222–228.4. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44:276–287.
Article5. Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010; 17:446–452.
Article6. Celik S, Erdogan S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol Cell Biochem. 2008; 312:39–46.
Article7. Correia S, Carvalho C, Santos MS, Proença T, Nunes E, Duarte AI, Monteiro P, Seiça R, Oliveira CR, Moreira PI. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008; 4:358–364.
Article8. Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000; 49:684–688.
Article9. Ferrannini E. Insulin resistance, insulin deficiency and the pathogenesis of diabetes mellitus. Clin Physiol. 1986; 6:311–317.
Article10. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article11. Gallwitz B, Kazda C, Kraus P, Nicolay C, Schernthaner G. Contribution of insulin deficiency and insulin resistance to the development of type 2 diabetes: nature of early stage diabetes. Acta Diabetol. 2013; 50:39–45.
Article12. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006; 97:1634–1658.
Article13. Hwang IK, Yi SS, Kim YN, Kim IY, Lee IS, Yoon YS, Seong JK. Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2008; 33:394–400.
Article14. Lappalainen Z, Lappalainen J, Oksala NKJ, Laaksonen DE, Khanna S, Sen CK, Atalay M. Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol (1985). 2009; 106:461–467.
Article15. Lindenau J, Noack H, Possel H, Asayama K, Wolf G. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000; 29:25–34.
Article16. Makar TK, Rimpel-Lamhaouar K, Abraham DG, Gokhale VS, Cooper AJL. Antioxidant defense systems in the brains of type II diabetic mice. J Neurochem. 1995; 65:287–291.
Article17. Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol. 2005; 187:37–44.
Article18. Mazzola PN, Terra M, Rosa AP, Mescka CP, Moraes TB, Piccoli B, Jacques CE, Dalazen G, Cortes MX, Coelho J, Dutra-Filho CS. Regular exercise prevents oxidative stress in the brain of hyperphenylalaninemic rats. Metab Brain Dis. 2011; 26:291–297.
Article19. Minamiyama Y, Bito Y, Takemura S, Takahashi Y, Kodai S, Mizuguchi S, Nishikawa Y, Suehiro S, Okada S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J Pharmacol Exp Ther. 2007; 320:535–543.
Article20. Nam SM, Kim YN, Yoo DY, Yi SS, Kim W, Hwang IK, Seong JK, Yoon YS. Hypothyroid states mitigate the diabetes-induced reduction of calbindin D-28k, calretinin, and parvalbumin immunoreactivity in type 2 diabetic rats. Neurochem Res. 2012; 37:253–260.
Article21. Nam SM, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Differential effects of treadmill exercise on cyclooxygenase-2 in the rat hippocampus at early and chronic stages of diabetes. Lab Anim Res. 2011; 27:189–195.
Article22. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Amsterdam: Elsevier;2007.23. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009; 106:8665–8670.
Article24. Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia. 2003; 46:940–948.
Article25. Siddiqui MR, Taha A, Moorthy K, Hussain ME, Basir SF, Baquer NZ. Amelioration of altered antioxidant status and membrane linked functions by vanadium and Trigonella in alloxan diabetic rat brains. J Biosci. 2005; 30:483–490.
Article26. Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007; 115:627–637.
Article27. Skjelbakken T, Valen G, Vaage J. Perfusing isolated rat hearts with hydrogen peroxide: an experimental model of cardiac dysfunction caused by reactive oxygen species. Scand J Clin Lab Invest. 1996; 56:431–439.
Article28. Smith SH, Kramer MF, Reis I, Bishop SP, Ingwall JS. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res. 1990; 67:1334–1344.
Article29. Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006; 8:517–528.
Article30. Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999; 16:93–112.
Article31. Suge R, Shimazu T, Hasegawa H, Inoue I, Hayashibe H, Nagasaka H, Araki N, Katayama S, Nomura M, Watanabe S. Cerebral antioxidant enzyme increase associated with learning deficit in type 2 diabetes rats. Brain Res. 2012; 1481:97–106.
Article32. Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011; 10:12.
Article33. Trachootham D, Lu W, Ogasawara MA, Rivera-Del Valle N, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008; 10:1343–1374.
Article34. Vora JP, Zimsen SM, Houghton DC, Anderson S. Evolution of metabolic and renal changes in the ZDF/Drt-fa rat model of type II diabetes. J Am Soc Nephrol. 1996; 7:113–117.
Article35. Wang J, Gong B, Zhao W, Tang C, Varghese M, Nguyen T, Bi W, Bilski A, Begum S, Vempati P, Knable L, Ho L, Pasinetti GM. Epigenetic mechanisms linking diabetes and synaptic impairments. Diabetes. 2014; 63:645–654.
Article36. Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991; 10:339–352.
Article37. Yi SS, Hwang IK, Chun MS, Kim YN, Kim IY, Lee IS, Seong JK, Yoon YS. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem Res. 2009; 34:851–858.
Article38. Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011; 36:117–128.
Article39. Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK. Melatonin improves Dgalactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012; 52:21–28.
Article40. Young IS, Tate S, Lightbody JH, McMaster D, Trimble ER. The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 1995; 18:833–840.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Cu Zn Levels on Superoxide Dismutase Activity in Erythrocytes from Patients with end Stage Renal Disease
- Effects of Vitamin E Supplementation on Oxidative Stress in Streptozotocin Induced Diabetic Rats: Investigation of Liver and Plasma
- Differential effects of treadmill exercise on cyclooxygenase-2 in the rat hippocampus at early and chronic stages of diabetes
- Effects of soybean isoflavone extract on the plasma lipid profiles and antioxidant enzyme activity in streptozotocin-induced diabetic rats
- Effect of Dietary Polyunsaturated / Saturated Fatty Acid on Membrane Lipid Peroxidation of Red Blood Cells and Hepatic Intracellular Organelles in Streptozotocin Induced Diabetec Rats