Infect Chemother.
2010 Oct;42(5):291-295. 10.3947/ic.2010.42.5.291.
Effects of Peroxisome Proliferator-Activated Receptor-gamma on the Production of Tumor Necrosis Factor-alpha in Stimulated Human Monocoytes
- Affiliations
-
- 1Catholic Research Institutes of Medical Science, College of Medicine, Catholic University of Korea, Seoul, Korea.
- 2Department of Internal Medicine, Division of Infectious Diseases, College of Medicine, Catholic University of Korea, Seoul, Korea. cmcjh@catholic.ac.kr
- KMID: 1806891
- DOI: http://doi.org/10.3947/ic.2010.42.5.291
Abstract
- BACKGROUND
We evaluated the effects of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) on the production of tumor necrosis factor-alpha (TNF-alpha) and expression of nuclear factor-kappaB (NF-kappaB) in stimulated THP-1 cells, a human monocyte cell line.
MATERIALS AND METHODS
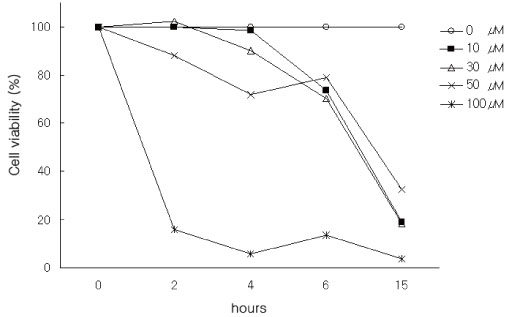
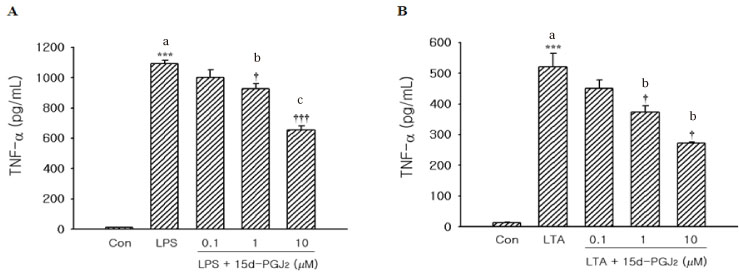
We evaluated the cytotoxic effect of 15-Deoxy-Delta(12,14)-prostaglandin J(2) (15d-PGJ(2)), one of natural PPAR-gamma ligands, using commercial cell proliferation assay. Cells were pretreated with 15d-PGJ(2) and then stimulated with lipopolysaccharide (LPS) or lipoteichoic acid (LTA). The amount of TNF-alpha was measured by using commercial ELISA method. NF-kappaB activation was evaluated by Western blot analysis.
RESULTS
15d-PGJ(2) showed dose-dependent cytotoxic effect on the tested cells after 4 hr of treatment. Stimulation of cells by LPS or LTA induced TNF-alpha production. TNF-alpha production was markedly decreased in the cells pretreated with 15d-PGJ(2) compared to cells treated only with LPS or LTA in a dose-dependent manner. Pretreatment of 15d-PGJ(2) reduced LPS or LTA induced NF-kappaB expression in the nuclear extracts of THP-1 cells.
CONCLUSION
15d-PGJ(2) pretreatment decreased TNF-alpha production from the THP-1 cells stimulated by LPS or LTA, and this assumed to be associated with inhibition of NF-kappaB activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi JH. Recent evidences of sepsis treatment. Infect Chemother. 2008. 40:67–75.
Article2. von Knethen A, Soller M, Brüne B. Peroxisome proliferator-activated receptor gamma (PPAR gamma) and sepsis. Arch Immunol Ther Exp (Warsz). 2007. 55:19–25.3. Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004. 53:Suppl 1. S43–S50.4. Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006. 116:581–589.5. Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, Inoue K, Hla T, Kondo M. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000. 270:400–405.
Article6. Balakumar P, Rose M, Singh M. PPAR ligands: are they potential agents for cardiovascular disorders? Pharmacology. 2007. 80:1–10.
Article7. Soller M, Tautenhahn A, Brüne B, Zacharowski K, John S, Link H, von Knethen A. Peroxisome proliferator-activated receptor gamma contributes to T lymphocyte apoptosis during sepsis. J Leukoc Biol. 2006. 79:235–243.
Article8. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.
Article9. Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008. 36:2849–2857.
Article10. Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003. 144:2201–2207.
Article11. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000. 14:1293–1307.
Article12. Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005. 23:393–399.
Article13. Han SY, Kim CH, Han KH, Cha DR, Kim HS. Combination treatment with retinoid and peroxisome proliferator-activated receptors (PPAR)-gamma agonist on streptozotocin-Induced diabetic nephropathy. Korean J Nephrol. 2007. 26:526–533.14. Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, Zhang SF. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit Care Med. 2005. 33:2309–2316.
Article15. Haraguchi G, Kosuge H, Maejima Y, Suzuki J, Imai T, Yoshida M, Isobe M. Pioglitazone reduces systematic inflammation and improves mortality in apolipoprotein E knockout mice with sepsis. Intensive Care Med. 2008. 34:1304–1312.
Article16. Boggild AK, Krudsood S, Patel SN, Serghides L, Tangpukdee N, Katz K, Wilairatana P, Liles WC, Looareesuwan S, Kain KC. Use of peroxisome proliferator-activated receptor gamma agonists as adjunctive treatment for Plasmodium falciparum malaria: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2009. 49:841–849.
Article17. Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-gamma. Blood. 2008. 112:4250–4258.
Article18. Chen YC, Shen SC, Tsai SH. Prostaglandin D(2) and J(2) induce apoptosis in human leukemia cells via activation of the caspase 3 cascade and production of reactive oxygen species. Biochim Biophys Acta. 2005. 1743:291–304.
Article19. Kim YA, Kim JH, Jung SJ, Gil JH, Ku NS, Park YS, Kim MS, Kim YK, Shin SY, Yoon HJ, Choi JY, Song YG, Kim JM. Effect of peroxisome proliferator-activated receptor gamma agonist on the incidence of sepsis in diabetic patients. Korean J Med. 2005. 69:Suppl 1. S168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory Effects of Fenofibrate with Inflammatory Response and Myocardiac Dysfunction in Lipopolysaccharide-stimulated Heart Tissues
- Anti-inflammatory effects of PPARgamma on human dental pulp cells
- Genipin Selectively Inhibits TNF-alpha-activated VCAM-1 But Not ICAM-1 Expression by Upregulation of PPAR-gamma in Human Endothelial Cells
- Fenofibrate, a peroxisome proliferator-activated receptor alpha-agonist, blocks lipopolysaccharide-induced inflammatory pathways in mouse liver
- Refocusing Peroxisome Proliferator Activated Receptor-alpha: A New Insight for Therapeutic Roles in Diabetes