Cancer Res Treat.
2004 Oct;36(5):308-314.
Effective Inhibition of Cancer Cell Growth by a Novel Tripartite Transfection Complex Containing Ribbon Antisense Molecules to hTR
- Affiliations
-
- 1Department of Medical Genetic Engineering, Keimyung University School of Medicine, Dongsan Medical Center, Daegu, Korea. jonggu@kmu.ac.kr
- 2WelGENE Inc., Daegu, Korea.
Abstract
- PURPOSE
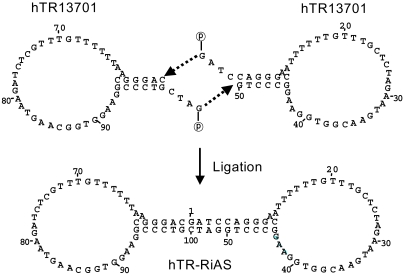
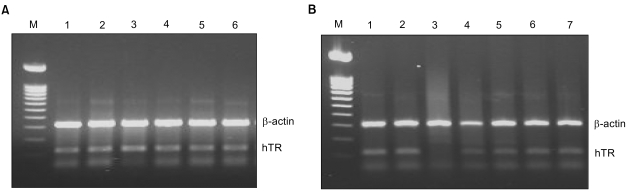
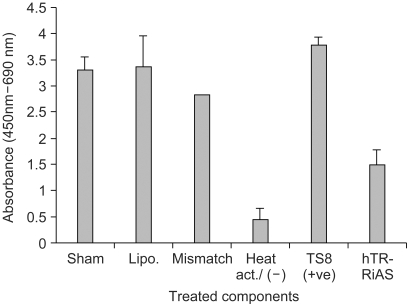
In the present study, ribbon antisense to the hTR RNA, a component of the telomerase complex, was employed to inhibit telomerase activity and cancer cell growth. MATERIALS AND METHODS: Ribbon antisense molecules to the human hTR gene (hTR-RiAS) were constructed and complexed with a short modified peptide and cationic liposomes to improve the cellular uptake of the antisense molecules. The DPL complexes containing hTR-RiAS were transfected into target cancer cells. Various assays were performed to confirm the effects of the hTR-RiAS on the gene expression and cell proliferation. RESULTS: When cancer cells were treated with hTR-RiAS, the cellular level of hTR mRNA was reduced by more than 95%, as shown by RT-PCR. Further, the telomerase activity was also affected by the antisense treatment. In contrast, both mismatched and scrambled oligonucleotides failed to reduce the levels of hTR mRNA and telomerase activity. When checked for cancer cell viability, hTR- RiAS inhibited cell growth by more than 70%, in a very rapid manner. The reduced cell viability was found to be due to apoptosis of cancer cells. CONCLUSION: These results show that hTR-RiAS is a powerful anticancer reagent, with the potential for broad efficacy to diverse malignant tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Blackburn EH. Structure and function of telomeres. Nature. 1991; 350:569–573. PMID: 1708110.
Article2. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460. PMID: 2342578.
Article3. Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai Ev, Futcher AB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992; 89:10114–10118. PMID: 1438199.
Article4. Urquidi V, Tarin D, Goodison S. Role of telomerase in cell senescence and oncogenesis. Annu Rev Med. 2000; 51:65–79. PMID: 10774453.
Article5. Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999; 274:7264–7271. PMID: 10066788.
Article6. Nugent CI, Bosco G, Ross LO, Evans SK, Salinger AP, Moore JK, Haber JE, Lundblad V. Telomere maintenance is dependent on activities required for end repair of double strand breaks. Curr Biol. 1998; 8:657–660. PMID: 9635193.7. Ishikawa T, Kamiyama M, Hisatomi H, Ichikawa Y, Momiyama N, Hamaguchi Y, Hasegawa S, Narita T, Shimada H. Telomerase enzyme activity and RNA expression in adriamycin-resistant human breast carcinoma MCF-7 cells. Cancer Lett. 1999; 141:187–194. PMID: 10454261.
Article8. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994; 266:2011–2015. PMID: 7605428.
Article9. Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. The RNA component of human telomerase. Science. 1995; 269:1236–1241. PMID: 7544491.
Article10. Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997; 90:785–795. PMID: 9288757.
Article11. Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997; 277:955–959. PMID: 9252327.
Article12. Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997; 275:973–977. PMID: 9020079.
Article13. Nakayama J, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997; 88:875–884. PMID: 9118230.
Article14. Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000; 60:4461–4467. PMID: 10969793.15. Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999; 96:14276–14281. PMID: 10588696.
Article16. Glukhov AI, Zimnik OV, Gordeev SA, Severin SE. Inhibition of telomerase activity of melanoma cells in vitro by antisense oligonucleotides. Biochem Biophys Res Commun. 1998; 248:368–371. PMID: 9675142.17. Norton JC, Piatyszek MA, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase activity by peptide nucleic acids. Nat Biotechnol. 1996; 14:615–619. PMID: 9630953.
Article18. Naka K, Yokozaki H, Yasui W, Tahara H, Tahara E, Tahara E. Effect of antisense human telomerase RNA transfection on the growth of human gastric cancer cell lines. Biochem Biophys Res Commun. 1999; 255:753–758. PMID: 10049783.
Article19. Kondo S, Tanaka Y, Kondo Y, Hitomi M, Barnett GH, Ishizaka Y, Liu J, Haqqi T, Nishiyama A, Villeponteau B, Cowell JK, Barna BP. Antisense telomerase treatment: induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1998; 12:801–811. PMID: 9657520.
Article20. Moon IJ, Lee Y, Kwak CS, Lee JH, Choi K, Schreiber AD, Park JG. Target site search and effective inhibition of leukaemic cell growth by a covalently closed multiple anti-sense oligo-nucleotide to c-myb. Biochem J. 2000; 346:295–303. PMID: 10677346.21. Moon IJ, Choi K, Choi YK, Kim JE, Lee Y, Schreiber AD, Park JG. Potent growth inhibition of leukemic cells by novel ribbon-type antisense oligonucleotides to c-myb1. J Biol Chem. 2000; 275:4647–4653. PMID: 10671493.
Article22. Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998; 16:3323–3330. PMID: 9681832.23. Kanazawa Y, Ohkawa K, Ueda K, Mita E, Takehara T, Sasaki Y, Kasahara A, Hayashi N. Hammerhead ribozyme-mediated inhibition of telomerase activity in extracts of human hepa tocellular carcinoma cells. Biochem Biophys Res Commun. 1996; 225:570–576. PMID: 8753802.24. Kosciolek BA, Kalantidis K, Tabler M, Rowley PT. Inhibition of telomerase activity in human cancer cells by RNA interference. Mol Cancer Ther. 2003; 2:209–216. PMID: 12657713.25. Kondo Y, Koga S, Komata T, Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000; 19:2205–2211. PMID: 10822370.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Genes Involved in Liver Cancer Cell Growth Using an Antisense Library of Phage Genomic DNA
- Development of Covalently Closed c-myb Antisense Oligonucleotides for Growth Inhibition of Leukemic Cells
- Growth Inhibition of Leukemic Cells by C-myb Antisense Oligos: Use of Simulational Target Site Search and Enhanced Delivery of Antisense Oligos by Cationic Liposomes
- The Effect of Ribbon-Type Antisense Oligodeoxynucleotides for Transforming Growth Factor-beta1 in Unilateral Ureteral Obstruction
- Effects of TGF-beta1 Ribbon Antisense on CCl4-induced Liver Fibrosis