Allergy Asthma Immunol Res.
2011 Jul;3(3):205-211. 10.4168/aair.2011.3.3.205.
Mesenchymal Stem Cell Transfer Suppresses Airway Remodeling in a Toluene Diisocyanate-Induced Murine Asthma Model
- Affiliations
-
- 1Division of Allergy and Respiratory Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. mdcspark@unitel.co.kr
- 2Division of Hematology, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 3Division of Hematology, Soonchunhyang University Seoul Hospital, Seoul, Korea.
- KMID: 1803327
- DOI: http://doi.org/10.4168/aair.2011.3.3.205
Abstract
- PURPOSE
Severe asthma is characterized by high medication requirements to maintain good disease control or by persistent symptoms despite high medication use. The transfer of bone marrow-derived mesenchymal stem cells (BMDMSCs) to the injured lungs is a possible treatment for severe asthma. This study investigated the therapeutic effects of BMDMSCs in airway remodeling and inflammation in an experimental toluene diisocyanate (TDI)-induced asthma animal model of severe asthma.
METHODS
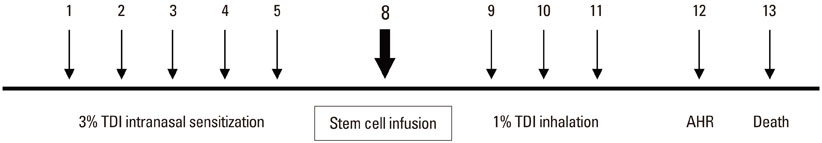
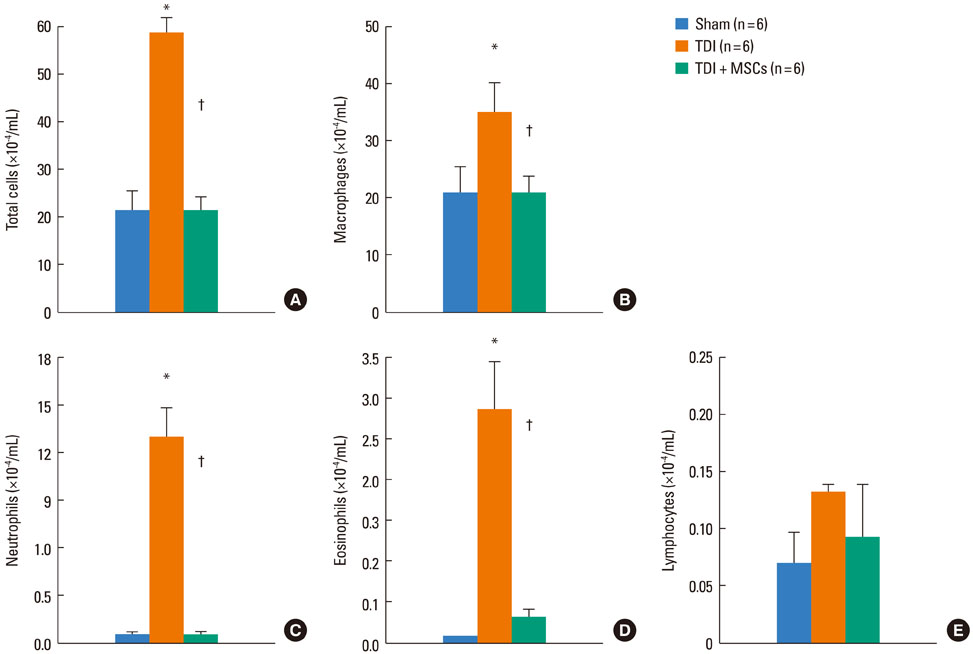
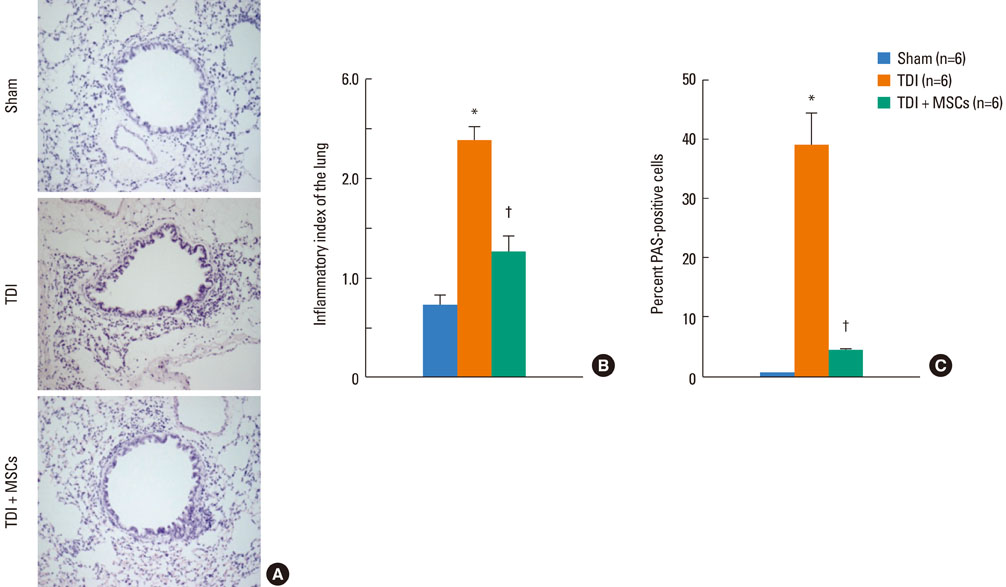
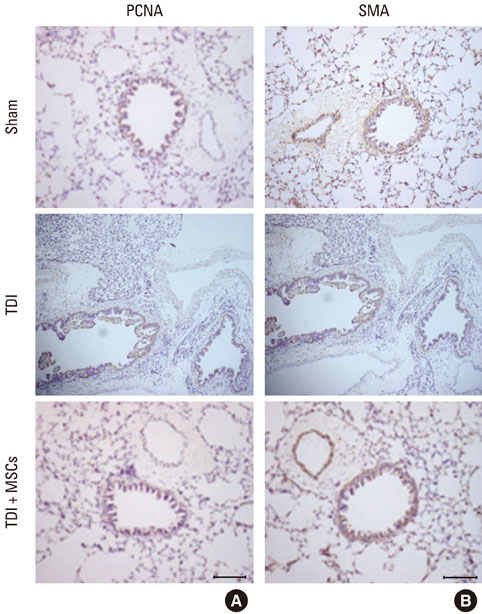
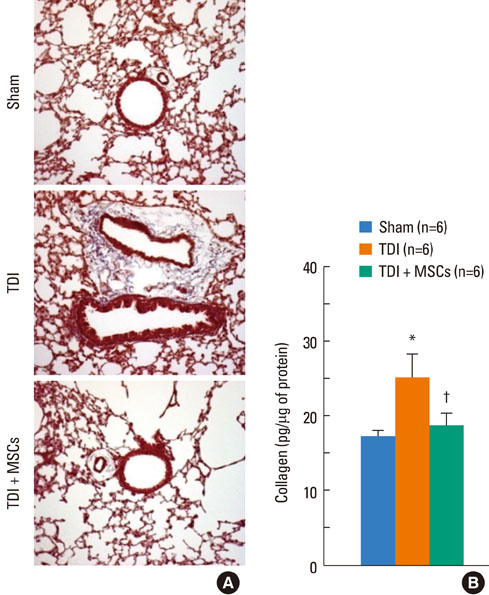
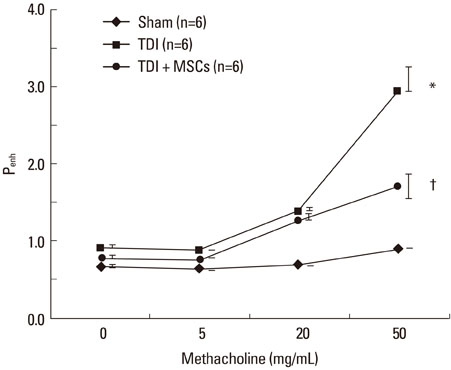
BMDMSCs were transferred into rats after TDI inhalation. Bronchoalveolar lavage (BAL) cell profiles, histological changes including an inflammatory index and goblet cell hyperplasia, and the airway response to methacholine using plethysmography were analyzed. Smooth muscle actin (SMA) and proliferating cell nuclear antigen (PCNA) protein expression were observed in lung tissue using immunohistochemical staining. The collagen content was measured in lung tissue sections and lung extracts using Masson's trichrome staining and an immunoassay kit.
RESULTS
The numbers of inflammatory cells in BAL fluid, histological inflammatory index, airway response to methacholine, number of goblet cells, and amount of collagen were increased in TDI-treated rats compared with sham rats (P=0.05-0.002). BMDMSC transfer significantly reduced the TDI-induced increase in the inflammatory index and numbers of eosinophils and neutrophils in BAL fluid to levels seen in sham-treated rats (P<0.05). BMDMSC transfer significantly reduced the number of goblet cells, collagen deposition, and immune staining for SMA and PCNA with concomitant normalization of the airway response to methacholine.
CONCLUSIONS
The systemic transfer of BMDMSCs effectively reduced experimental TDI-induced airway inflammation and remodeling and airway hyperreactivity.
Keyword
MeSH Terms
-
Actins
Airway Remodeling
Animals
Asthma
Bronchoalveolar Lavage
Collagen
Eosinophils
Goblet Cells
Hyperplasia
Immunoassay
Inflammation
Inhalation
Lung
Mesenchymal Stromal Cells
Methacholine Chloride
Models, Animal
Muscle, Smooth
Neutrophils
Plethysmography
Proliferating Cell Nuclear Antigen
Rats
Salicylamides
Stem Cells
Toluene
Toluene 2,4-Diisocyanate
Actins
Collagen
Methacholine Chloride
Proliferating Cell Nuclear Antigen
Salicylamides
Toluene
Toluene 2,4-Diisocyanate
Figure
Cited by 1 articles
-
Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma
Jung Hur, Ji Young Kang, Young Kyoon Kim, Sook Young Lee, Sora Jeon, Yourha Kim, Chan Kwon Jung, Chin Kook Rhee
J Korean Med Sci. 2020;35(23):e188. doi: 10.3346/jkms.2020.35.e188.
Reference
-
1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008. 31:143–178.2. Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003. 123:417S–422S.3. American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000. 162:2341–2351.4. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998. 339:1194–1200.5. Mealey FH, Kenyon NJ, Avdalovic MV, Louie S. Difficult-to-control asthma in adults. Am J Med. 2007. 120:760–763.6. Thomas M, Haughney J, Price D. Cost effectiveness of asthma management strategies. Pharmacoeconomics. 2002. 20:789.7. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003. 167:1360–1368.8. Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005. 116:544–549.9. Park SW, Park JS, Lee YM, Lee JH, Jang AS, Kim DJ, Hwangbo Y, Uh ST, Kim YH, Park CS. Differences in radiological/HRCT findings in eosinophilic bronchitis and asthma: implication for bronchial responsiveness. Thorax. 2006. 61:41–47.10. ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. "Refractory" eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004. 170:601–605.11. Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. The AMPUL Study Group. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am J Respir Crit Care Med. 1999. 159:1043–1051.12. Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000. 162:1308–1313.13. Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, Park SK, Lee YK, Won JH, Kim YH, Park CS. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010. 11:16.14. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.15. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998. 16:155–162.16. Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998. 95:1142–1147.17. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.18. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003. 100:8407–8411.19. Ahn MH, Park BJ, Kwon JH, An SH, Park JW, Jang AS, Rhim T, Park CS. Asp-Tyr-Leu-Lys tetrapeptide inhibits airway inflammation in toluene-2,4-diisocyanate-induced asthma mice. Clin Exp Allergy. 2008. 38:1025–1032.20. Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004. 117:5655–5664.21. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997. 156:766–775.22. Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009. 1:10–18.23. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006. 12:574–579.24. Chan-Yeung M. Occupational asthma. Chest. 1990. 98:148S–161S.25. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005. 33:145–152.26. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004. 172:1266–1272.27. Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L131–L141.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of occupational asthma with systemic illness due to toluene diisocyanate and diphenylmethane diisocyanate
- Role of Matrix Metalloproteinase in the Pathogenesis of Bronchial Asthma
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- A Murine Model of Toluene Diisocyanate-induced Contact Hypersensitivity
- A case of bronchial asthma induced by toluene diisocyanate(TDI) in the external environment