Yonsei Med J.
2008 Aug;49(4):592-600. 10.3349/ymj.2008.49.4.592.
The Inhibition of Insulin-stimulated Proliferation of Vascular Smooth Muscle Cells by Rosiglitazone Is Mediated by the Akt-mTOR-P70S6K Pathway
- Affiliations
-
- 1Cardiology Division, Department of Internal Medicine, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. kchwang@yuhs.ac
- 2Cardiovascular Research Institute, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Washington University in St. Louis School of Medicine, 606 S. Euclid Ave., St. Louis, MO 63108, USA.
- KMID: 1793194
- DOI: http://doi.org/10.3349/ymj.2008.49.4.592
Abstract
- PURPOSE
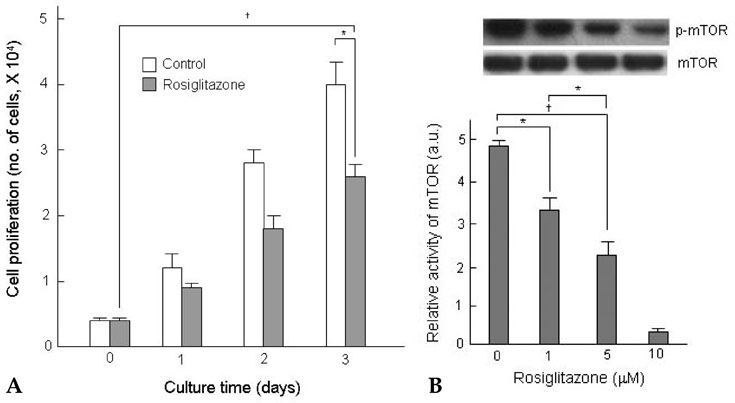
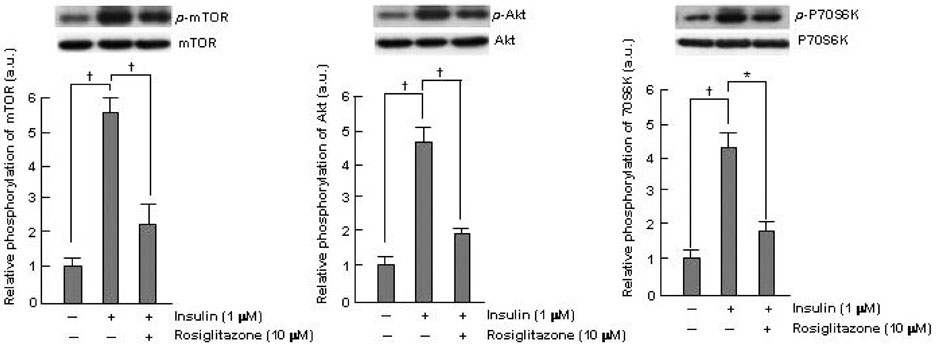
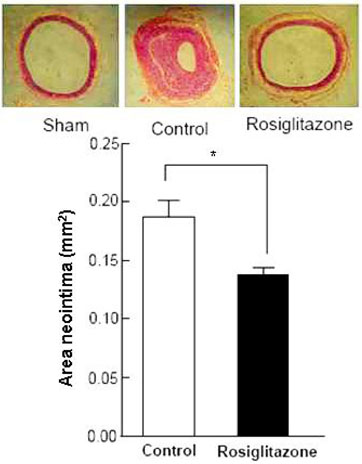
Thiazolidinediones (TZDs) are known to inhibit the proliferation of vascular smooth muscle cell (VSMC) by increasing the activity of p27(Kip1) and retinoblastoma protein (RB). However, the upstream signaling mechanisms associated with this pathway have not been elucidated. The Akt-mTOR-P70S6 kinase pathway is the central regulator of cell growth and proliferation, and increases cell proliferation by inhibiting the activities of p27(Kip1) and retinoblastoma protein (RB). Therefore, we hypothesized in this study that rosiglitazone inhibits VSMC proliferation through the inhibition of the Akt-TOR-P70S6K signaling pathway. MATERIALS and METHODS: Rat aortic smooth muscle cells (RAoSMCs) were treated with 10microM of rosiglitazone 24 hours before the addition of insulin as a mitogenic stimulus. Western blot analysis was performed to determine the inhibitory effect of rosiglitazone treatment on the Akt-mTOR-P70S6K signaling pathway. Carotid balloon injury was also performed in Otsuka Long-Evans Tokushima Fatty (OLETF) diabetic rats that were pretreated with 3 mg/kg of rosiglitazone. RESULTS: Western blot analysis demonstrated significant inhibition of activation of p-Akt, p-m-TOR, and p-p70S6K in cells treated with rosiglitazone. The inhibition of the activation of the p-mTOR-p-p70S6K pathway seemed to be mediated by both the upstream PI3K pathway and MEK-ERK complex. CONCLUSION: The inhibitory effect of rosiglitazone on RAoSMC proliferation in vitro and in vivo is mediated by the inhibition of the Akt-mTOR-P70S6K pathway.
MeSH Terms
-
Animals
Cell Proliferation/drug effects
Cells, Cultured
Cytoprotection/drug effects
Enzyme Activation/drug effects
Insulin/*pharmacology
Male
Mitogen-Activated Protein Kinase Kinases/antagonists & inhibitors/metabolism
Muscle, Smooth, Vascular/drug effects/*metabolism
Myocytes, Smooth Muscle/drug effects/*metabolism
Phosphorylation
Protein Kinase Inhibitors/pharmacology
Protein Kinases/*metabolism
Proto-Oncogene Proteins c-akt/antagonists & inhibitors/*metabolism
Rats
Ribosomal Protein S6 Kinases, 70-kDa/*metabolism
Signal Transduction/drug effects
Thiazolidinediones/*pharmacology
Figure
Cited by 1 articles
-
Anti-Proliferative Effects of Rutin on OLETF Rat Vascular Smooth Muscle Cells Stimulated by Glucose Variability
Sung Hoon Yu, Jae Myung Yu, Hyung Joon Yoo, Seong Jin Lee, Dong Hyun Kang, Young Jung Cho, Doo Man Kim
Yonsei Med J. 2016;57(2):373-381. doi: 10.3349/ymj.2016.57.2.373.
Reference
-
1. Law RE, Meehan WP, Xi XP, Graf K, Wuthrich DA, Coats W, et al. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996. 98:1897–1905.
Article2. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.3. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998. 391:79–82.4. Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999. 19:2094–2104.
Article5. Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000. 101:235–238.
Article6. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995. 270:12953–12956.
Article7. Bruemmer D, Law RE. Thiazolidinedione regulation of smooth muscle cell proliferation. Am J Med. 2003. 115:Suppl 8A. 87S–92S.
Article8. Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004. 27:2654–2660.
Article9. Sehgal SN. Rapamune (Sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995. 17:660–665.
Article10. Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996. 98:2277–2283.
Article11. Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005. 17:158–166.
Article12. Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst). 2004. 3:927–934.
Article13. Indolfi C, Torella D, Cavuto L, Davalli AM, Coppola C, Esposito G, et al. Effects of balloon injury on neointimal hyperplasia in streptozotocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet-transplanted rats. Circulation. 2001. 103:2980–2986.
Article14. Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, et al. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001. 59:866–875.
Article15. Hafizi S, Wang X, Chester AH, Yacoub MH, Proud CG. ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004. 287:H1232–H1238.
Article16. Lee KH, Lim S, Kang SM, Kim DH, Cho HK, Chung JH, et al. Antiproliferative mechanisms of raxofelast (IRFI-016) in H2O2-stimulated rat aortic smooth muscle cells. Eur J Pharmacol. 2004. 484:119–125.
Article17. Farese RV. Insulin-sensitive phospholipid signaling systems and glucose transport. Update II. Exp Biol Med (Maywood). 2001. 226:283–295.
Article18. Taha C, Klip A. The insulin signaling pathway. J Membr Biol. 1999. 169:1–12.
Article19. Olefsky JM, Saltiel AR. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab. 2000. 11:362–368.20. Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003. 8:65–79.
Article21. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003. 115:577–590.
Article22. Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995. 76:412–417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “The Inhibition of Insulin-stimulated Proliferation of Vascular Smooth Muscle Cells by Rosiglitazone Is Mediated by the Akt-mTOR-P70S6K Pathway” by Park S, et al. (Yonsei Med J 2008 Aug;49(4):592-600)
- Far-Infrared Irradiation Decreases Proliferation in Basal and PDGFStimulated VSMCs Through AMPKMediated Inhibition of mTOR/p70S6K Signaling Axis
- Activation of AMPK by Telmisartan Decreases Basal and PDGF-stimulated VSMC Proliferation via Inhibiting the mTOR/p70S6K Signaling Axis
- Integrins Mediating Adhesion and Proliferation of ADP-stimulated Vascular Smooth Muscle Cells
- Insulin Enhances Suppressive Effect of Lipopolysaccharide on Glucose-induced Proliferation of Vascular Smooth Muscle Cells