J Korean Med Sci.
2020 Sep;35(35):e289. 10.3346/jkms.2020.35.e289.
Activation of AMPK by Telmisartan Decreases Basal and PDGF-stimulated VSMC Proliferation via Inhibiting the mTOR/p70S6K Signaling Axis
- Affiliations
-
- 1Department of Pharmacology, Yeungnam University College of Medicine, Daegu, Korea
- 2Department of Molecular Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2506085
- DOI: http://doi.org/10.3346/jkms.2020.35.e289
Abstract
- Background
Telmisartan, an angiotensin II type 1 receptor blocker (ARB), is widely used to treat hypertension by blocking the renin-angiotensin-aldosterone system. Although abnormal proliferation of vascular smooth muscle cells (VSMCs) is a well-established contributor to the development of various vascular diseases, such as atherosclerosis, the effect of telmisartan on VSMC proliferation and its mechanism of action have not been fully revealed. Herein, we investigated the molecular mechanism whereby telmisartan inhibits rat VSMC proliferation.
Methods
We measured VSMC proliferation by MTT assay, and performed inhibitor studies and western blot analyses using basal and platelet-derived growth factor (PDGF)-stimulated rat VSMCs. To elucidate the role of AMP-activated protein kinase (AMPK), we introduced dominant-negative (dn)-AMPKα1 gene into VSMCs.
Results
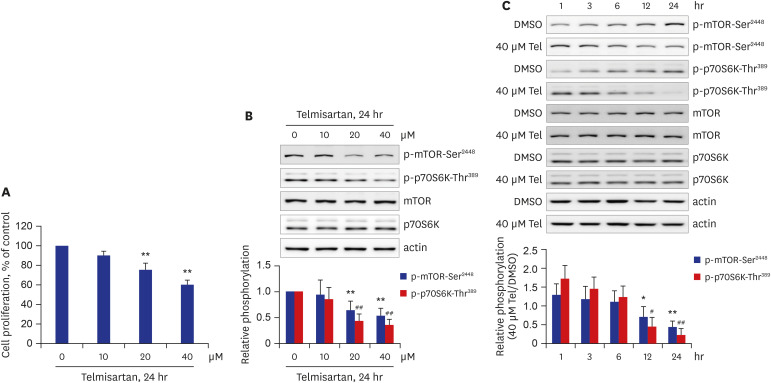
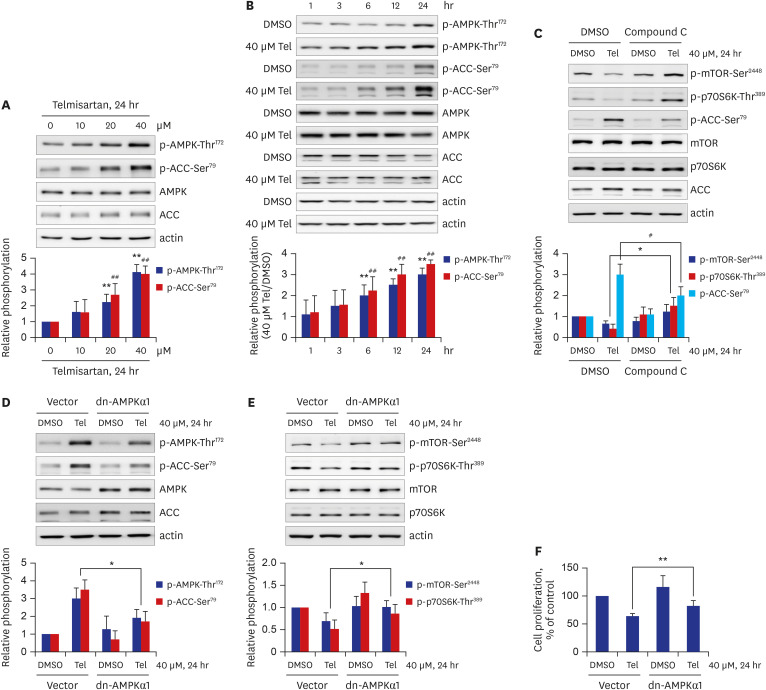
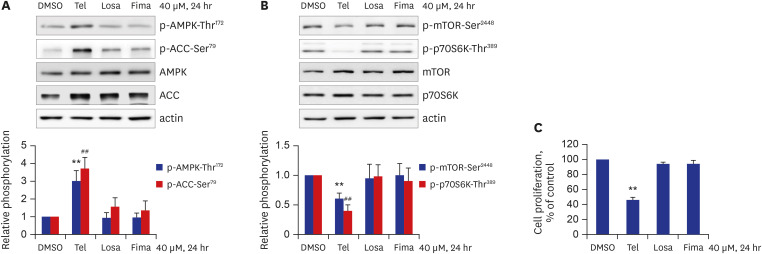
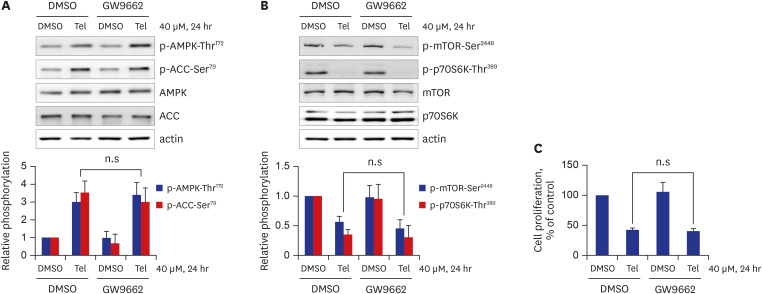
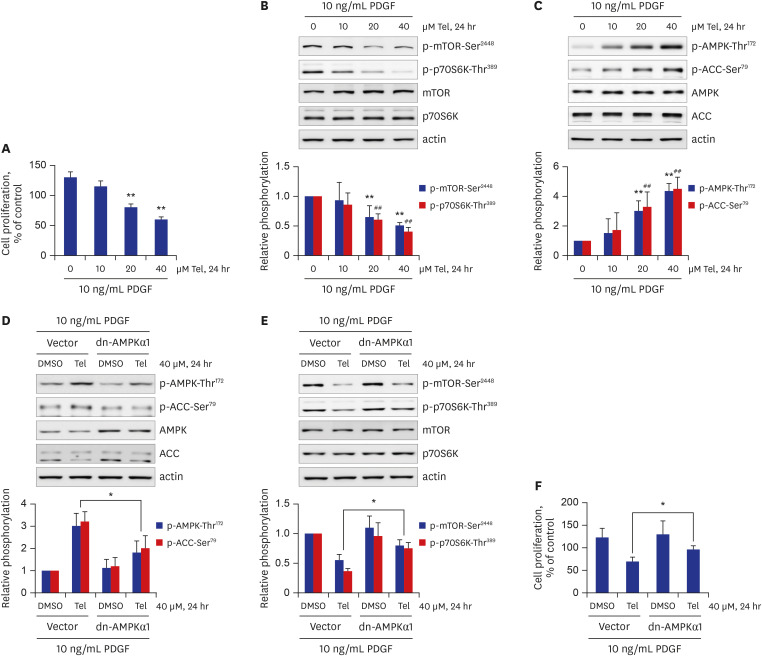
Telmisartan decreased VSMC proliferation, which was accompanied by decreased phosphorylations of mammalian target of rapamycin (mTOR) at Ser2448 (p-mTOR-Ser2448 ) and p70 S6 kinase (p70S6K) at Thr389 (p-p70S6K-Thr389 ) in dose- and time-dependent manners. Telmisartan dose- and time-dependently increased phosphorylation of AMPK at Thr172 (p-AMPK-Thr172 ). Co-treatment with compound C, a specific AMPK inhibitor, or ectopic expression of the dn-AMPKα1 gene, significantly reversed telmisartan-inhibited VSMC proliferation, p-mTOR-Ser2448 and p-p70S6K-Thr389 levels. Among the ARBs tested (including losartan and fimasartan), only telmisartan increased p-AMPK-Thr172 and decreased p-mTOR-Ser2448 , p-p70S6K-Thr389 , and VSMC proliferation. Furthermore, GW9662, a specific and irreversible peroxisome proliferator-activated receptor γ (PPARγ) antagonist, did not affect any of the telmisartan-induced changes. Finally, telmisartan also exhibited inhibitory effects on VSMC proliferation by increasing p-AMPK-Thr172 and decreasing p-mTOR-Ser2448 and p-p70S6K-Thr389 in a PDGF-induced in vitro atherosclerosis model.
Conclusion
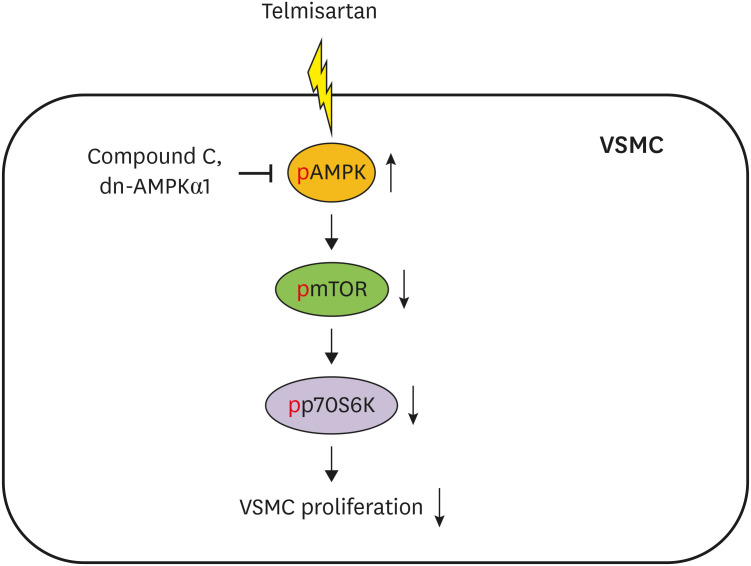
These results demonstrated that telmisartan-activated AMPK inhibited basal and PDGF-stimulated VSMC proliferation, at least in part, by downregulating the mTOR/p70S6K signaling axis in a PPARγ-independent manner. These observations suggest that telmisartan could be used to treat arterial narrowing diseases such as atherosclerosis and restenosis.
Keyword
Figure
Reference
-
1. Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004; 43(5):993–1002. PMID: 15007034.2. Song KH, Park JH, Jo I, Park JY, Seo J, Kim SA, et al. Telmisartan attenuates hyperglycemia-exacerbated VCAM-1 expression and monocytes adhesion in TNFα-stimulated endothelial cells by inhibiting IKKβ expression. Vascul Pharmacol. 2016; 78:43–52. PMID: 26455386.
Article3. Hwang YJ, Cho DH. Activation of AMPK/proteasome/MLCK degradation signaling axis by telmisartan inhibits VSMC contractility and vessel contraction. Biochem Biophys Res Commun. 2020; 524(4):853–860. PMID: 32046856.
Article4. Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012; 95(2):194–204. PMID: 22467316.
Article5. Rivard A, Andrés V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histol Histopathol. 2000; 15(2):557–571. PMID: 10809377.6. Yamamoto K, Ohishi M, Ho C, Kurtz TW, Rakugi H. Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009; 54(6):1353–1359. PMID: 19822796.7. Destro M, Cagnoni F, Dognini GP, Galimberti V, Taietti C, Cavalleri C, et al. Telmisartan: just an antihypertensive agent? A literature review. Expert Opin Pharmacother. 2011; 12(17):2719–2735. PMID: 22077832.
Article8. Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013; 65(2):809–848. PMID: 23487168.
Article9. Miura S, Karnik SS, Saku K. Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst. 2011; 12(1):1–7. PMID: 20603272.
Article10. Papadopoulos N, Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Aspects Med. 2018; 62:75–88. PMID: 29137923.
Article11. Shawky NM, Segar L. Sulforaphane inhibits platelet-derived growth factor-induced vascular smooth muscle cell proliferation by targeting mTOR/p70S6kinase signaling independent of Nrf2 activation. Pharmacol Res. 2017; 119:251–264. PMID: 28212891.
Article12. Dong X, Hu H, Fang Z, Cui J, Liu F. CTRP6 inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration. Biomed Pharmacother. 2018; 103:844–850. PMID: 29710500.
Article13. Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012; 95(2):156–164. PMID: 22406749.
Article14. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992; 326(4):242–250. PMID: 1727977.15. Schwartz SM, deBlois D, O'Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995; 77(3):445–465. PMID: 7641318.16. Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002; 8(11):1249–1256. PMID: 12411952.
Article17. Hong MK. Restenosis following coronary angioplasty: current status. Korean J Intern Med (Korean Assoc Intern Med). 2001; 16(2):51–55.18. Li Z, Li Y, Jia Y, Ding B, Yu J. Rab1A knockdown represses proliferation and promotes apoptosis in gastric cancer cells by inhibition of mTOR/p70S6K pathway. Arch Biochem Biophys. 2020; 685:108352. PMID: 32240637.
Article19. Li J, Liu W, Hao H, Wang Q, Xue L. Rapamycin enhanced the antitumor effects of doxorubicin in myelogenous leukemia K562 cells by downregulating the mTOR/p70S6K pathway. Oncol Lett. 2019; 18(3):2694–2703. PMID: 31404320.
Article20. Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY, Liao X, et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018; 14:656–668. PMID: 29175753.
Article21. Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015; 14(4):473–480. PMID: 25590164.
Article22. You G, Long X, Song F, Huang J, Tian M, Xiao Y, et al. Metformin activates the AMPK-mTOR pathway by modulating lncRNA TUG1 to induce autophagy and inhibit atherosclerosis. Drug Des Devel Ther. 2020; 14:457–468.23. Wu H, Song A, Hu W, Dai M. The anti-atherosclerotic effect of paeonol against vascular smooth muscle cell proliferation by up-regulation of autophagy via the AMPK/mTOR signaling pathway. Front Pharmacol. 2018; 8:948. PMID: 29354055.
Article24. Zhao Y, Shang F, Shi W, Zhang J, Zhang J, Liu X, et al. Angiotensin II receptor type 1 antagonists modulate vascular smooth muscle cell proliferation and migration via AMPK/mTOR. Cardiology. 2019; 143(1):1–10. PMID: 31307032.
Article25. Jin Z, Tan Q, Sun B. Telmisartan ameliorates vascular endothelial dysfunction in coronary slow flow phenomenon (CSFP). Cell Biochem Funct. 2018; 36(1):18–26. PMID: 29314204.
Article26. Markan U, Pasupuleti S, Pollard CM, Perez A, Aukszi B, Lymperopoulos A. The place of ARBs in heart failure therapy: is aldosterone suppression the key? Ther Adv Cardiovasc Dis. 2019; 13:1753944719868134. PMID: 31401939.
Article27. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008; 372(9644):1174–1183. PMID: 18757085.28. Diener HC. Preventing stroke: the PRoFESS, ONTARGET, and TRANSCEND trial programs. J Hypertens Suppl. 2009; 27(5):S31–6. PMID: 19587553.
Article29. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994; 91(15):7355–7359. PMID: 8041794.
Article30. Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001; 276(41):37731–37734. PMID: 11459852.31. Zídek V, Mlejnek P, Simáková M, Silhavy J, Landa V, Kazdová L, et al. Tissue-specific peroxisome proliferator activated receptor gamma expression and metabolic effects of telmisartan. Am J Hypertens. 2013; 26(6):829–835. PMID: 23426788.32. Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997; 46(8):1319–1327. PMID: 9231657.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Far-Infrared Irradiation Decreases Proliferation in Basal and PDGFStimulated VSMCs Through AMPKMediated Inhibition of mTOR/p70S6K Signaling Axis
- Mesoglycan attenuates VSMC proliferation through activation of AMP-activated protein kinase and mTOR
- Influence of Endothelin-1 on Cultured Vascular Smooth Muscle Cell Proliferation
- The Inhibition of Insulin-stimulated Proliferation of Vascular Smooth Muscle Cells by Rosiglitazone Is Mediated by the Akt-mTOR-P70S6K Pathway
- Cilostazol Inhibits Vascular Smooth Muscle Cell Proliferation and Reactive Oxygen Species Production through Activation of AMP-activated Protein Kinase Induced by Heme Oxygenase-1